How to find the sequence of Nusinersen?
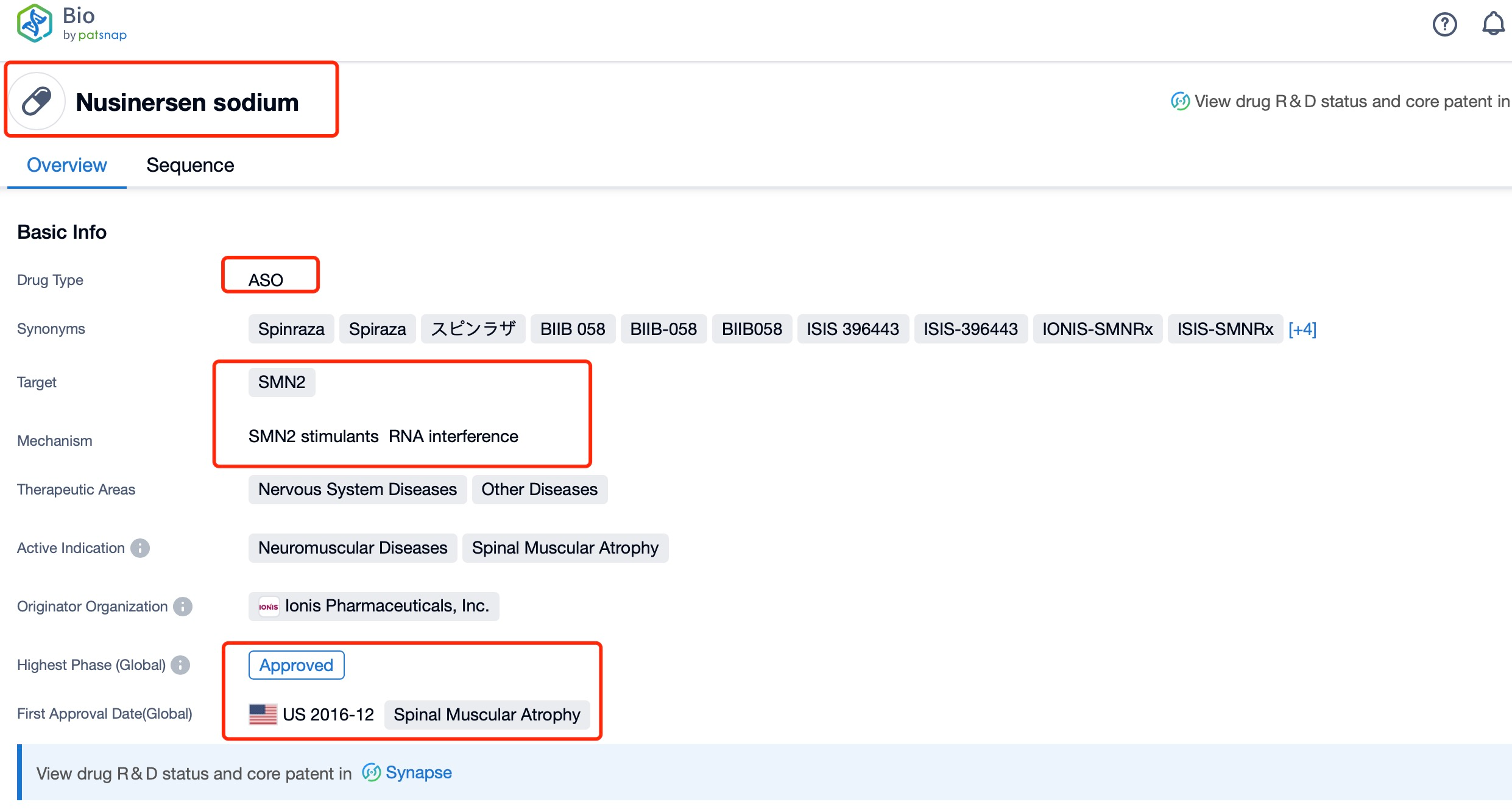
Nusinersen, developed by Biogen in collaboration with Ionis Pharmaceuticals, is an antisense oligonucleotide that targets the survival motor neuron 2 (SMN2) gene. The SMN2 gene is a nearly identical copy of the survival motor neuron 1 (SMN1) gene, which is critical for the survival of motor neurons. Mutations or deletions in the SMN1 gene lead to spinal muscular atrophy (SMA), a severe and often fatal neuromuscular disorder characterized by the loss of motor neurons and progressive muscle weakness. Nusinersen is indicated for the treatment of SMA in pediatric and adult patients, aiming to increase the production of full-length SMN protein by modulating the splicing of the SMN2 pre-mRNA.
Summary of Research Progress of Nusinersen
Nusinersen works by binding to a specific site on the SMN2 pre-mRNA, known as the intronic splicing silencer N1 (ISS-N1), which normally inhibits the inclusion of exon 7 in the mature mRNA. By binding to this site, nusinersen blocks the inhibitory effect of ISS-N1, promoting the inclusion of exon 7 and resulting in the production of a full-length, functional SMN protein. This mechanism of action helps to compensate for the deficiency in SMN protein caused by mutations in the SMN1 gene. Nusinersen is administered intrathecally, directly into the cerebrospinal fluid, which allows for targeted delivery to the central nervous system where the motor neurons are located. The drug has been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of SMA, and it has shown significant efficacy in clinical trials.
Globally, the competition in the SMA market is growing, with several companies developing novel therapies. Nusinersen faces competition from other antisense oligonucleotides, such as risdiplam (Evrysdi) developed by Roche and PTC Therapeutics, which is an orally administered small molecule that also aims to increase the production of full-length SMN protein. Additionally, gene therapy approaches, such as Zolgensma (onasemnogene abeparvovec) developed by Novartis, offer a one-time treatment that delivers a functional copy of the SMN1 gene to motor neurons. Despite this competition, nusinersen remains a significant player in the SMA market due to its established safety profile and proven efficacy in a wide range of SMA patients.
Clinical research on nusinersen has demonstrated its efficacy and safety in treating SMA. The Phase 3 clinical trials, such as the ENDEAR and CHERISH studies, showed that nusinersen could significantly improve motor function and survival in infants and children with SMA. The drug was generally well-tolerated, with common side effects including injection site reactions, respiratory infections, and constipation. Ongoing and future trials aim to further evaluate the long-term safety and efficacy of nusinersen, as well as explore its potential in combination with other therapies to achieve better outcomes. The SHINE open-label extension study continues to monitor the long-term effects of nusinersen in patients who have completed the initial clinical trials.
Sequence Characteristics of Nusinersen
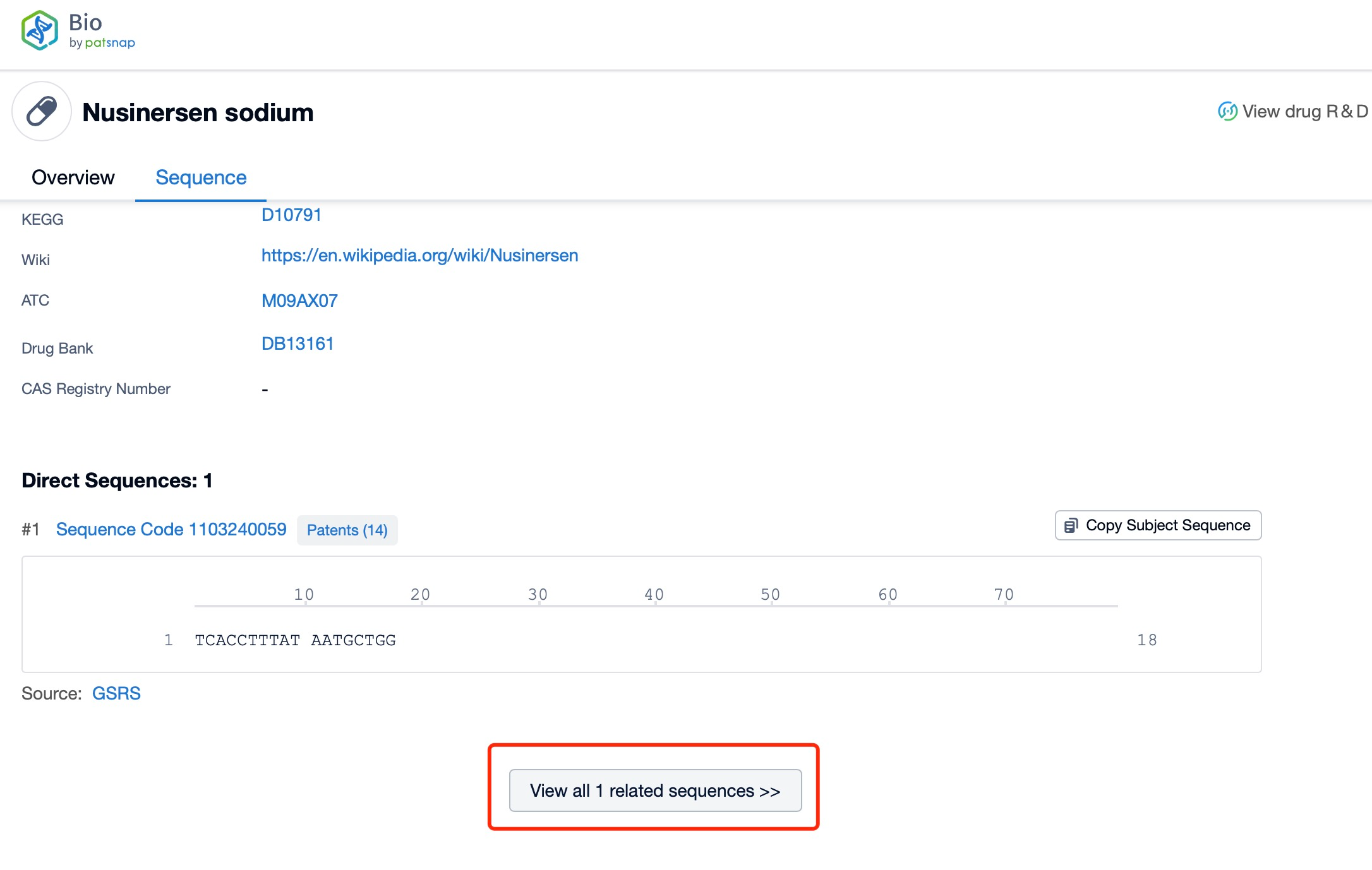
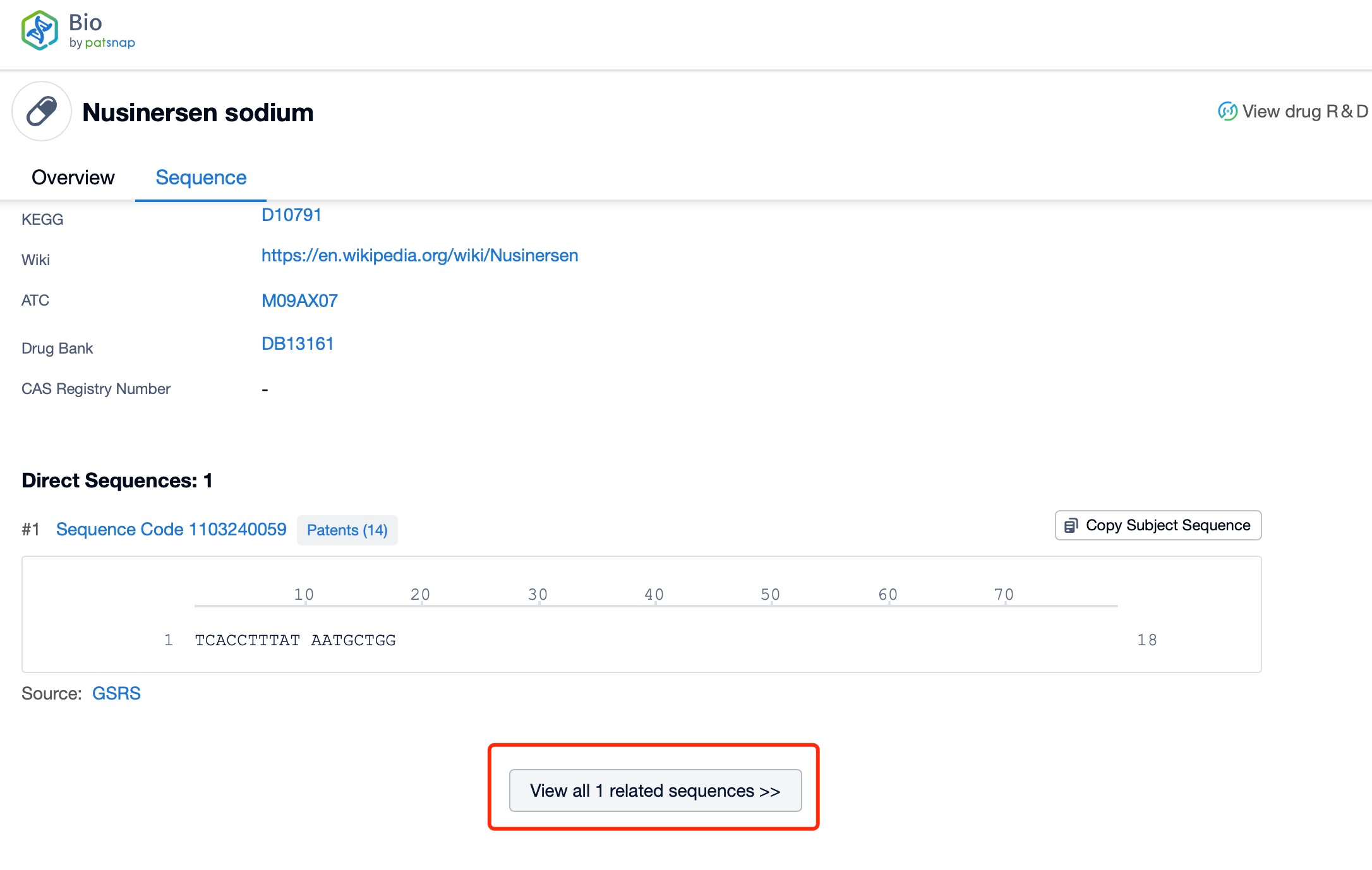
Nusinersen is a 18-nucleotide phosphorothioate-modified oligonucleotide. The sequence of nusinersen is specifically designed to bind to the intronic splicing silencer N1 (ISS-N1) on the SMN2 pre-mRNA. This sequence is optimized to ensure high specificity and efficiency in binding to the target site, which is crucial for the drug's therapeutic efficacy and safety. The precise sequence of nusinersen is a proprietary information, but it is known to be highly complementary to the target ISS-N1, allowing for effective modulation of splicing and the production of full-length SMN protein.
Chemical Modification and Species of Nusinersen
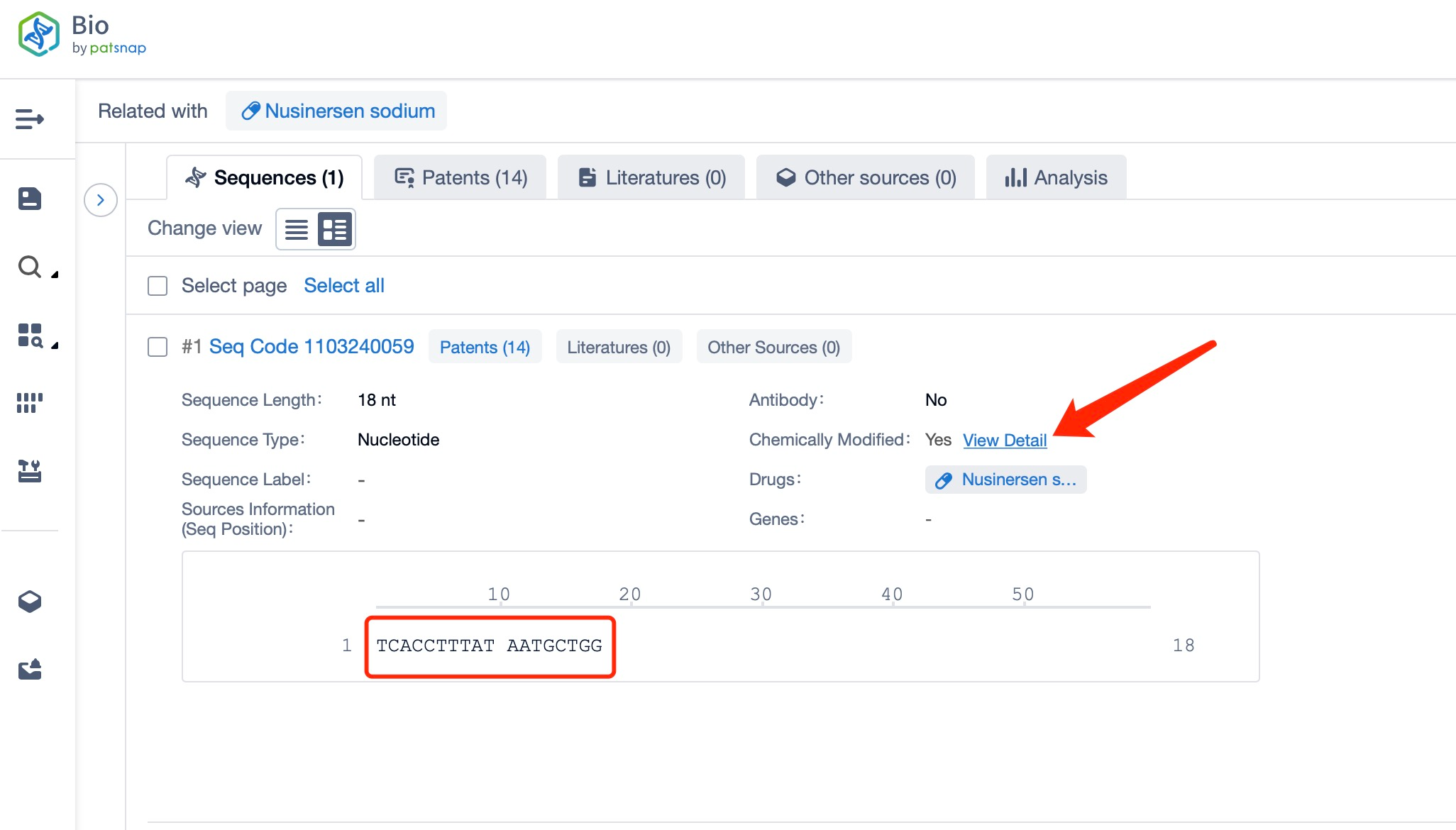
The chemical modifications in nusinersen include phosphorothioate linkages. The phosphorothioate backbone replaces one of the non-bridging oxygen atoms in the phosphate group with a sulfur atom, enhancing the stability of the oligonucleotide and protecting it from nuclease degradation. This modification is crucial for the drug's ability to reach and bind to the target pre-mRNA in the central nervous system. The phosphorothioate backbone also improves the pharmacokinetic properties of the drug, such as its half-life and tissue distribution, which are important for its therapeutic effectiveness.
The phosphorothioate modifications in nusinersen provide several key advantages. First, they significantly enhance the stability of the oligonucleotide, allowing it to remain active in the cellular environment for a longer period. This increased stability is crucial for the drug's effectiveness in binding to and modulating the splicing of the SMN2 pre-mRNA. Second, the phosphorothioate backbone improves the pharmacokinetic profile of the drug, increasing its half-life and bioavailability. This means that the drug can be administered less frequently, which is beneficial for patient convenience and compliance. Third, the phosphorothioate modifications reduce the risk of off-target effects by enhancing the specificity of the oligonucleotide for its target pre-mRNA.
The role of the phosphorothioate modifications in nusinersen is multifaceted. They protect the oligonucleotide from degradation by nucleases, ensuring that it can reach its target in the central nervous system. They also enhance the binding affinity of the oligonucleotide to the ISS-N1 site on the SMN2 pre-mRNA, ensuring efficient and specific modulation of splicing. Additionally, the phosphorothioate modifications improve the pharmacokinetic properties of the drug, such as its half-life and tissue distribution, which are essential for its therapeutic effectiveness. These modifications also reduce the immunogenicity of the oligonucleotide, minimizing the risk of adverse immune responses.
Summary
In summary, nusinersen represents a significant advancement in antisense technology and the treatment of spinal muscular atrophy (SMA). Its mechanism of action, involving the modulation of splicing of the SMN2 pre-mRNA, has shown promising results in increasing the production of full-length SMN protein and improving motor function and survival in affected patients. Despite facing competition from other antisense oligonucleotides and gene therapies, nusinersen offers a unique advantage with its established safety profile and proven efficacy in a wide range of SMA patients. Future research may focus on improving delivery methods and exploring combination therapies to enhance its clinical utility. The sequence characteristics and chemical modifications of nusinersen, including its phosphorothioate backbone, contribute to its stability, specificity, and efficacy, making it a valuable tool in the management of SMA. However, the need for regular intrathecal injections and the management of side effects will continue to be important considerations in its clinical use.
How to find the sequence of an ASO?
In Patsnap Bio, you can find the sequence and latest research and development advances of all ASOs.
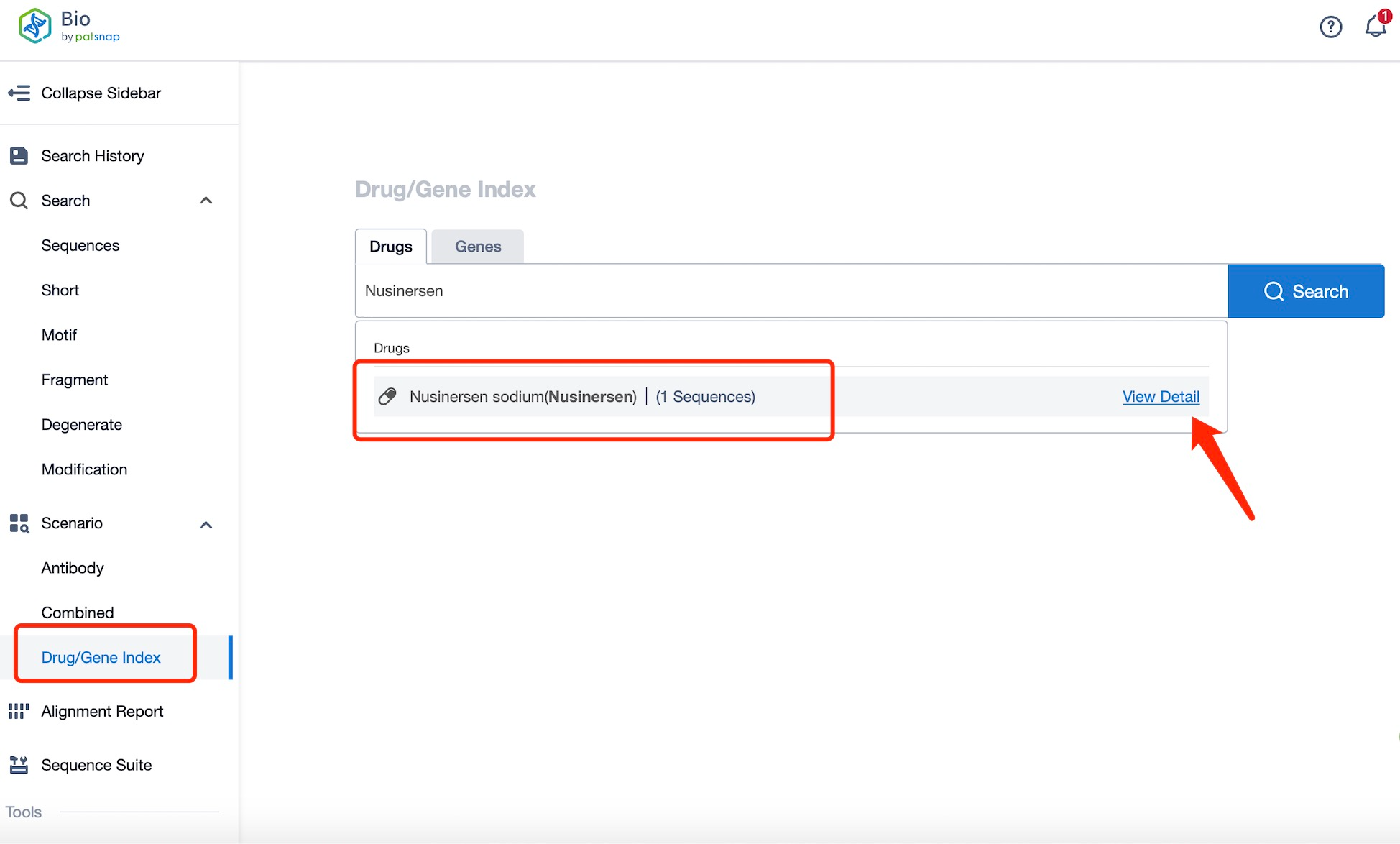
Taking Nusinersen as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' Nusinersen ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of Nusinersen.
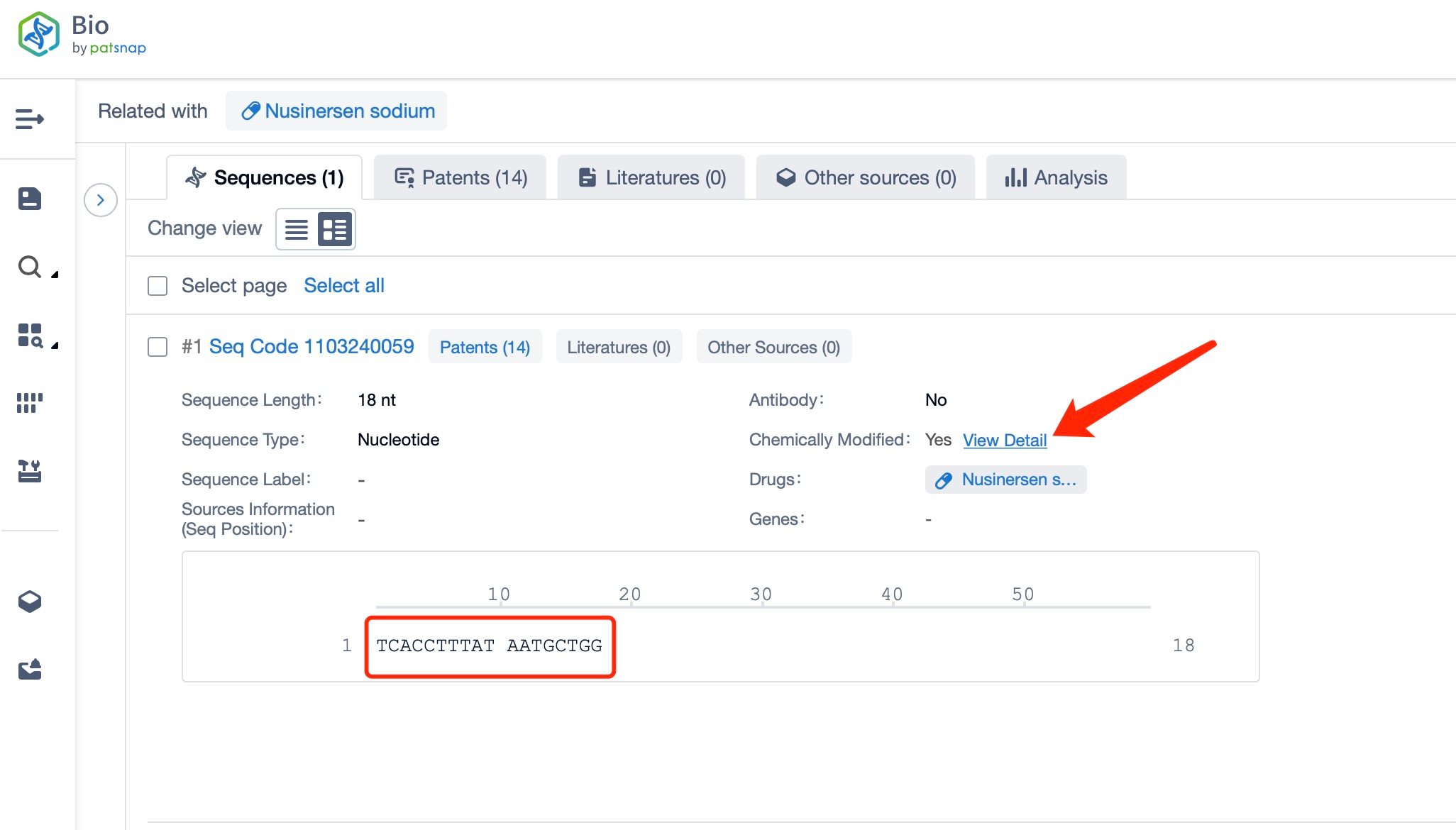
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
Clicking on the sequence name will provide you with all the basic information of that sequence.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.