I-Mab Unveils Latest Phase 1 Givastomig Results at ESMO 2024
I-Mab (NASDAQ: IMAB) (the "Company"), a global biotech enterprise headquartered in the United States, dedicated to developing innovative immunotherapies specifically for cancer treatment, announced the presentation of a poster focusing on promising top-line results from its active Phase 1 clinical trial (NCT04900818) of givastomig. Givastomig is a pioneering first-in-class bispecific antibody immunostimulant targeting Claudin18.2 (CLDN18.2) and 4-1BB, tested on patients with advanced malignancies, notably gastric cancers, including gastroesophageal carcinoma (GEC). This presentation occurred at the European Society for Medical Oncology (ESMO) Congress 2024, held in Barcelona, Spain.
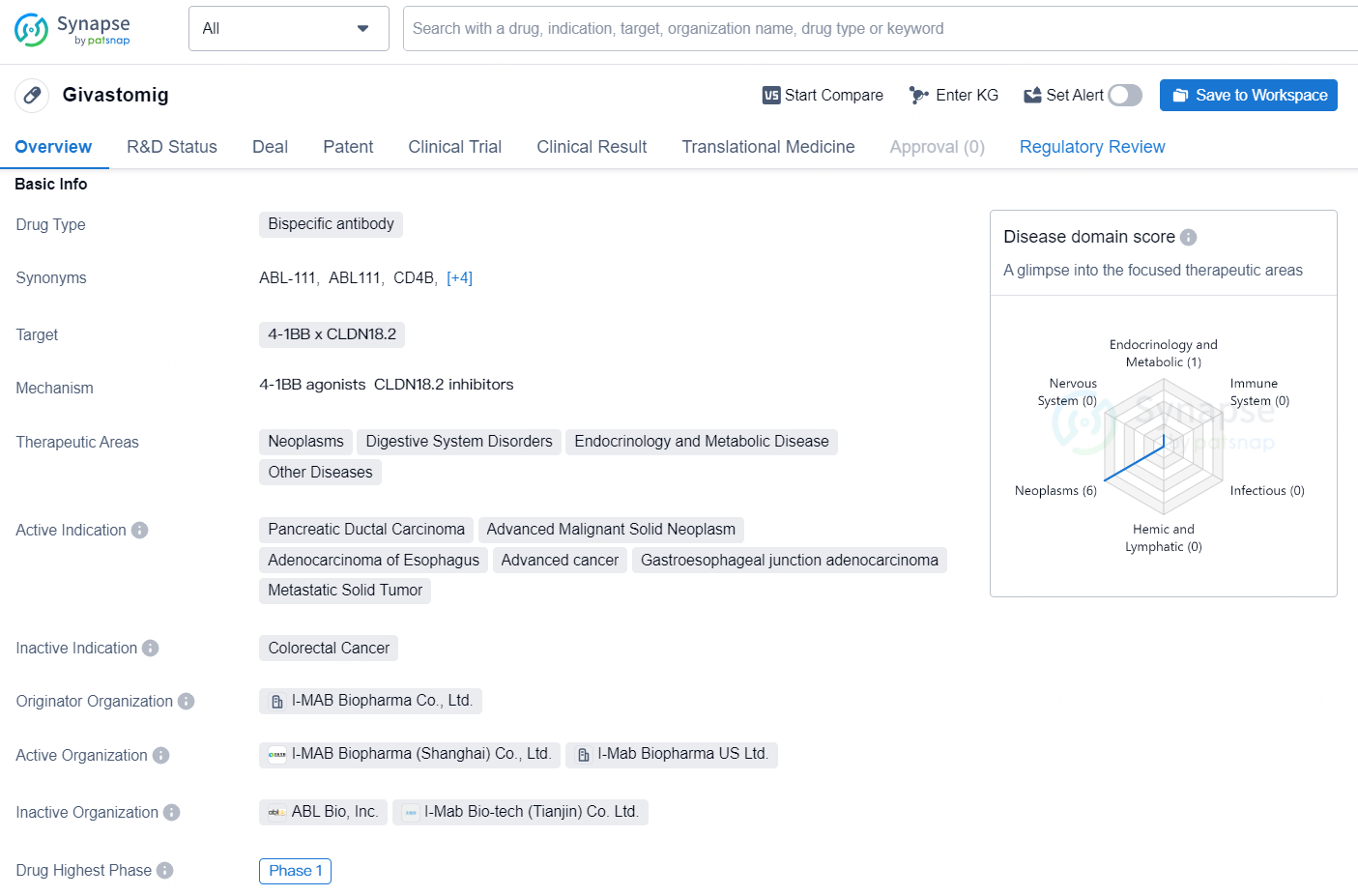
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Givastomig (TJ033721 / ABL111) is a bispecific antibody that targets Claudin 18.2-positive tumor cells and conditionally activates T cells via the 4-1BB pathway within the tumor microenvironment where Claudin 18.2 is present. Givastomig distinguishes itself from other Claudin 18.2-targeting therapies by demonstrating localized and conditional activation in nonclinical studies, even in tumors with minimal CLDN18.2 expression. Additionally, it has shown a favorable safety profile in clinical trial participants thus far.
"We believe givastomig could potentially serve as a frontline treatment option for patients with gastric cancers. Data presented at ESMO 2024 indicate that givastomig has consistently shown monotherapy efficacy in heavily pre-treated patients, including those with varying levels of Claudin 18.2 expression, while maintaining an excellent overall safety profile. This combination of attributes reinforces our belief that givastomig has the potential to be a distinctive, leading therapy in its class," stated Dr. Phillip Dennis, MD, PhD, Chief Medical Officer of I-Mab. "The initial evaluation of givastomig as a frontline treatment for gastric cancers is currently in progress. The Phase 1b dose expansion study will assess givastomig in combination with standard-of-care treatment, nivolumab plus chemotherapy. We remain highly optimistic about the program and anticipate sharing the study's results in the latter half of 2025."
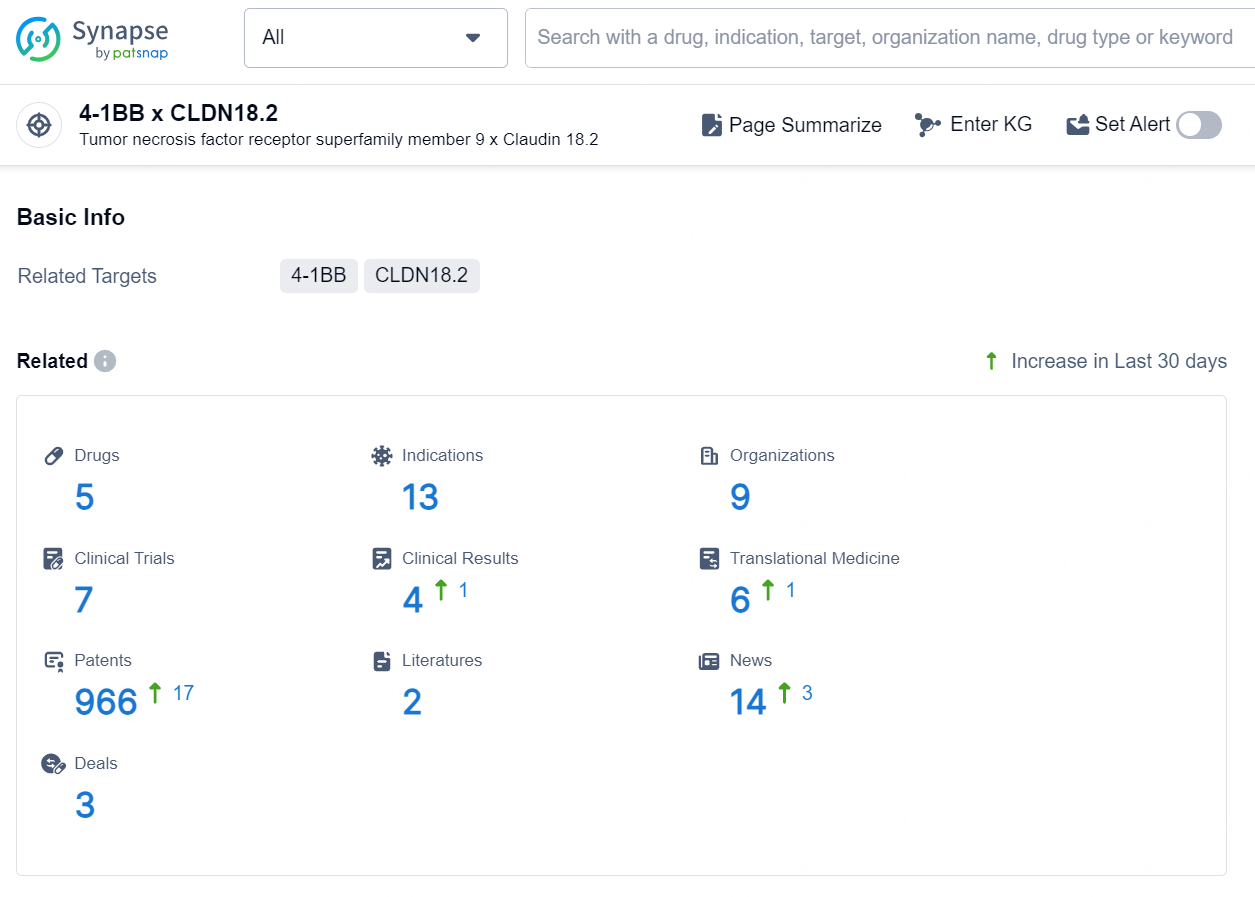
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 18, 2024, there are 1 investigational drug for the 4-1BB x CLDN18.2 targets, including 20 indications, 2 R&D institutions involved, with related clinical trials reaching 29, and as many as 1018 patents.
Givastomig is a bispecific antibody drug that targets 4-1BB x CLDN18.2 and is intended for use in a range of therapeutic areas, including neoplasms, digestive system disorders, endocrinology and metabolic diseases, and other diseases. The drug is actively indicated for the treatment of pancreatic ductal carcinoma, advanced malignant solid neoplasm, adenocarcinoma of the esophagus, advanced cancer, gastroesophageal junction adenocarcinoma, and metastatic solid tumors.