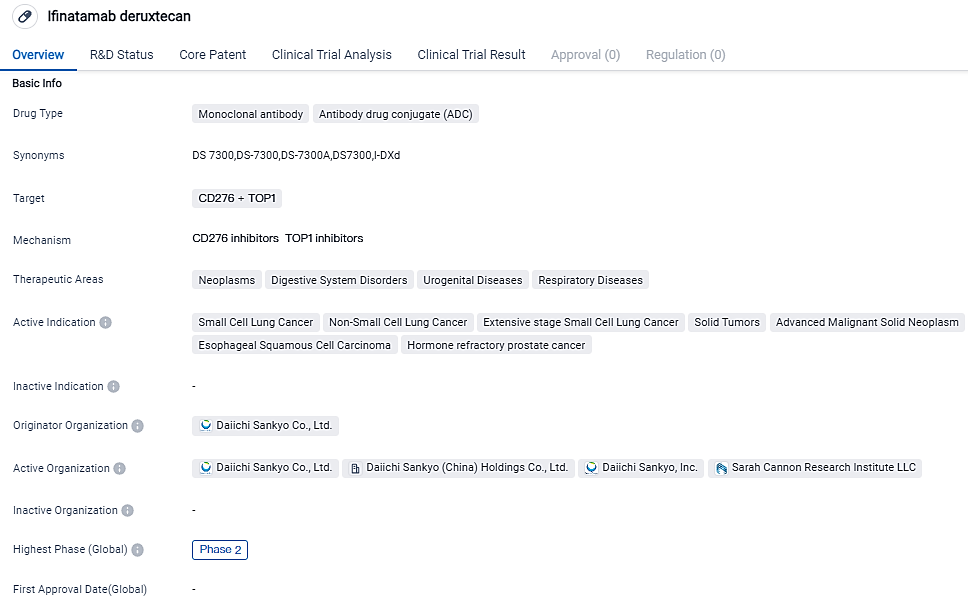
Ifinatamab Deruxtecan Continues to Demonstrate Durable Responses in Patients with Advanced Small Cell Lung Cancer in Early Trial
Updated results from a subgroup analysis of a phase 1/2 trial showed that ifinatamab deruxtecan (I-DXd) continues to demonstrate durable responses in patients with heavily pretreated advanced small cell lung cancer. These data were presented today during an oral presentation at the 2023 World Conference on Lung Cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Ifinatamab deruxtecan is a specifically engineered potential first-in-class B7-H3 directed antibody drug conjugate designed using Daiichi Sankyo’s proprietary DXd ADC technology.
Lung cancer is the second most common cancer worldwide and SCLC represents about 15% of all cases. Approximately 65% of all SCLC tumors have a moderate-to-high expression of B7-H3, which is associated with disease progression and lower survival.
Tumor reduction seen with ifinatamab deruxtecan was observed across a broad range of B7-H3 protein expression levels and no apparent trend of correlation between clinical efficacy parameters and B7-H3 protein expression was observed.
“With limited effective treatment options beyond traditional chemotherapy and immunotherapy, small cell lung cancer can be difficult to treat,” said Melissa Johnson, MD, Director, Lung Cancer Research, Sarah Cannon Research Institute. “The high response rate, along with the fact that all patients except one experienced a reduction in tumor size with ifinatamab deruxtecan, is promising.”
“In addition to the response rate seen with ifinatamab deruxtecan, we are further encouraged by the median overall survival seen in these patients at approximately one year,” said Mark Rutstein, MD, Global Head, Oncology Clinical Development, Daiichi Sankyo. “Additional evaluation of this B7-H3 directed antibody drug conjugate is underway in our ongoing phase 2 trial in patients with previously treated extensive-stage small cell lung cancer and we look forward to learning these results.”
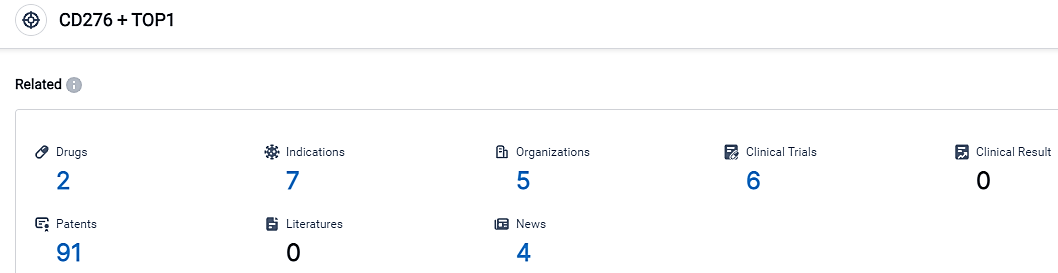
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 14, 2023, there are 2 investigational drugs for the CD276 and TOP1 target, including 7 applicable indications,5 R&D institutions involved, with related clinical trials reaching 6,and as many as 91 patents.
Ifinatamab deruxtecan (I-DXd) is an investigational potential first-in-class B7-H3 directed ADC. Designed using Daiichi Sankyo’s proprietary DXd ADC technology. Ifinatamab deruxtecan is being evaluated in a global development program, a phase 2 monotherapy trial in patients with previously treated extensive-stage SCLC, and a phase 1/2 first-in-human trial in collaboration with Sarah Cannon Research Institute.