Bristol Myers Squibb's trial LPA1 Antagonist reduced lung function decline in Progressive Pulmonary Fibrosis patients in a phase 2 study
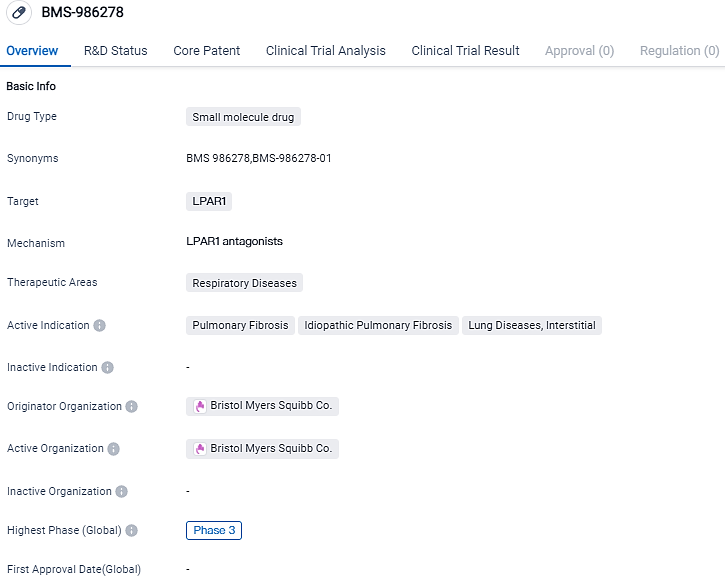
Bristol Myers Squibb disclosed outcomes from a Phase 2 examination of BMS-986278, a potential first-in-class, administrated orally, and is an adversary to the lysophosphatidic acid receptor 1 (LPAR1). This was in utilization for patients experiencing progressive pulmonary fibrosis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The findings of the study demonstrated that the bimonthly application of 60 mg of BMS-986278 within a span of 26 weeks diminished the falling percentage of predicted forced vital capacity by 69% in contrast to the placebo. These findings are to be unveiled during the Abstracts Leading to Evolution in Respiratory Medicine Trials phase 1 at the European Respiratory Society 2023 International Congress.
" People suffering from pulmonary fibrosis urgently need fresh approaches to tackle this destructive disease, which has a median survival rate of 3-5 years," stated Professor Tamera J. Corte, a respiratory physician consultant, interstitial lung disease director, and clinical trial investigator.
Professor Corte also highlighted the promising nature of BMS-986278, "The findings from the Phase 2 study on progressive pulmonary fibrosis, proving its consistent effectiveness whether co-administered with antifibrotic therapy or not and displaying a positive tolerability profile, further emphasize the potential of BMS-986278. As it acts as a testament to the progress made in the field, we're striving to discover more novel standards of care."
The multinational Phase 2 clinical trial was randomized and tested the effects of 30 mg or 60 mg of BMS-986278 or a similar placebo, administered orally twice a day for 26 weeks, on parallel patient cohorts suffering from both idiopathic pulmonary fibrosis and PPF. The unrestricted inclusion of antifibrotics in the IPF treatment regimen and/or certain immunosuppressives in the PPF therapy was permissible.
The primary objective of the study was to observe any alterations in ppFVC from the initial state to the 26th week in patients with IPF. Rate of change in ppFVC from baseline through 26 weeks in the PPF cohort was a key secondary endpoint of the study and was assessed based on two prespecified estimands.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
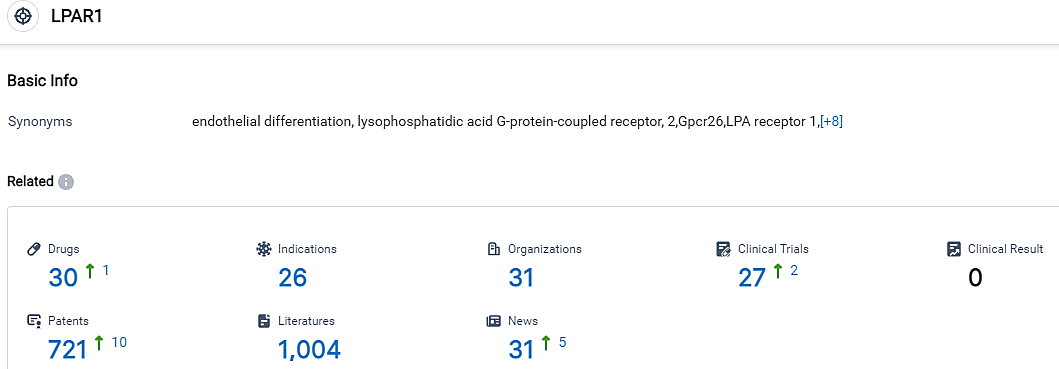
According to the data provided by the Synapse Database, As of September 14, 2023, there are 30 investigational drugs for the LPAR1 target, including 26 applicable indications,31 R&D institutions involved, with related clinical trials reaching 27,and as many as 721 patents.
BMS-986278 is a potential first-in-class, oral, small molecule lysophosphatidic acid receptor 1 (LPA1) antagonist currently being evaluated as a novel antifibrotic treatment for patients with idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. Increased LPA levels and activation of LPA1are involved in the pathogenesis of pulmonary fibrosis. A preclinicalin vitroandin vivostudy found that antagonizing LPA1may be beneficial in treating lung injury and fibrosis.