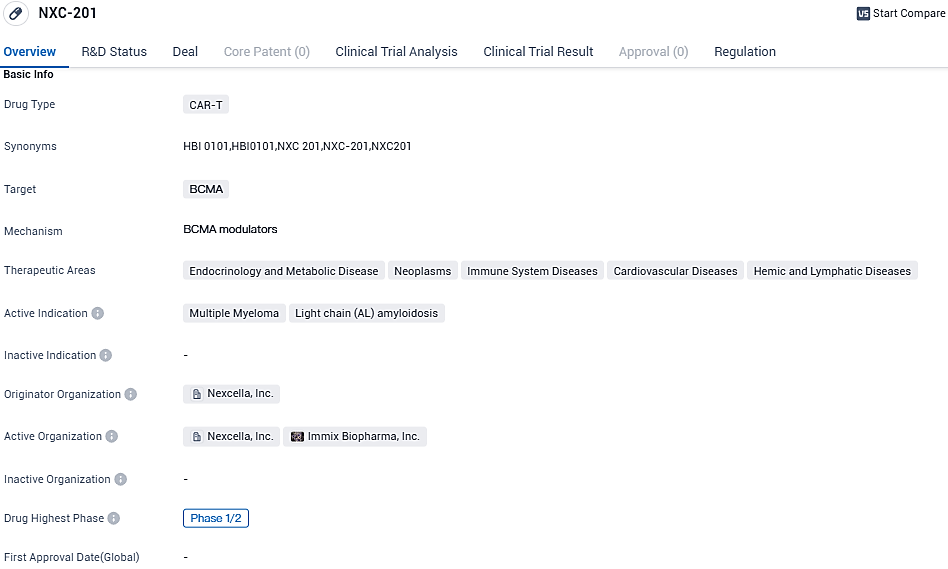
Immix Biopharma's IND for CAR-T NXC-201 approved by FDA, enabling treatment for U.S. patients
Immix Biopharma, Inc., a company specializing in the clinical phase of biopharmaceuticals and committed to pioneering personalized treatments for cancer and immunity, revealed today that the FDA has approved the Investigational New Drug submission for BCMA CAR-T NXC-201 (formerly HBI0101).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
With the approval in place, NEXICART-2 is set to extend the trials of NXC-201 in patients with relapsed/refractory AL Amyloidosis in the U.S. Its positive tolerability could potentially be utilized for further expansion into autoimmune applications.
"We're enthusiastic about the promising clinical evidence of NXC-201 so far, and the fact that a number of top-notch U.S facilities are on track to register patients in the following months," commented Ilya Rachman, MD PhD, Chief Executive Officer at Immix Biopharma. "There is no sanctioned treatment available as of now for relapsed/refractory AL Amyloidosis."
Outside of the U.S, NXC-201 has been administered to 72 patients. The relapsed/refractory AL Amyloidosis dosage scales were: 150 x 106 (n=1), 450 x 106 (n=2), 800 x 106 (n=6) CAR+T cells, showcasing a 100% general response rate (9/9) (with a median of 6 lines of previously used therapy). Dosage scales for relapsed/refractory multiple myeloma were: 150 x 106 (n=6), 450 x 106 (n=7), 800 x 106 (n=50) CAR+T cells, showing a 95% overall response rate with a median follow up period of 11.9 months.
Gabriel Morris, Chief Financial Officer at Immix Biopharma, attributed this IND approval in accordance with their initially communicated schedules to their top-tier cell-therapy expert team. He stated, “The encouraging tolerability record of NXC-201, including the capacity to mitigate neurotoxicity, may allow for its use beyond AL Amyloidosis into autoimmune disorders.”
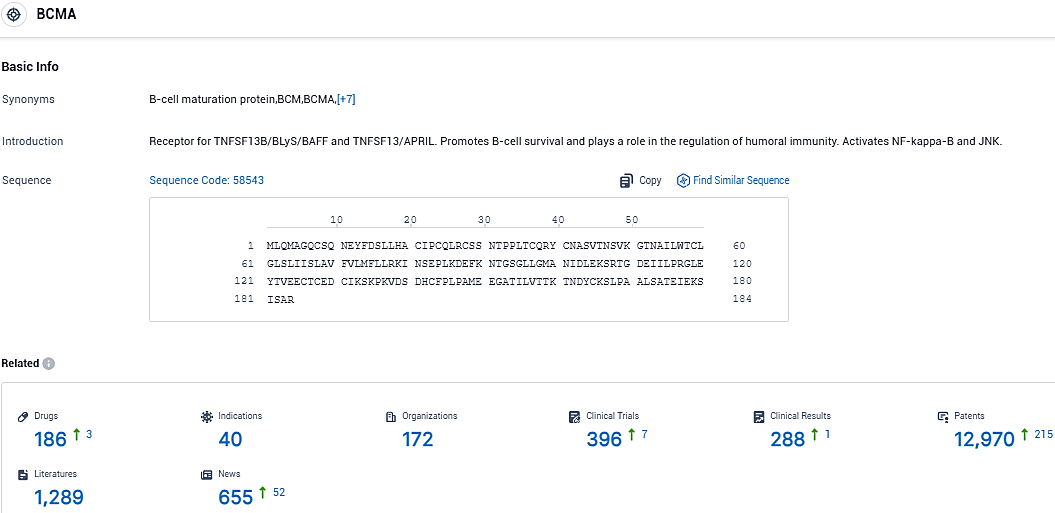
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 28, 2023, there are 186 investigational drugs for the BCMA target, including 40 indications, 172 R&D institutions involved, with related clinical trials reaching 396, and as many as 12970 patents.
NXC-201 is a BCMA-targeted investigational chimeric antigen receptor T cell therapy that is being studied in a comprehensive clinical development program for the treatment of patients with relapsed/refractory AL amyloidosis, relapsed/refractory multiple myeloma, and potentially expanding into autoimmune indications: systemic lupus erythematosus, myasthenia gravis, and multiple sclerosis. NXC-201 has been awarded Orphan Drug Designation by the FDA in both AL Amyloidosis and multiple myeloma.