Immune-Onc Presents Positive Phase 1b Results for IO-202 in CMML at 2024 ASH
Immune-Onc Therapeutics, Inc., a privately held clinical-stage biopharmaceutical firm developing innovative immunology and oncology treatments by focusing on myeloid cell inhibitory receptors, has unveiled the latest data from its Phase 1b expansion cohort. The study assesses IO-202, a pioneering anti-LILRB4 antibody, in conjunction with azacitidine (AZA) for patients with chronic myelomonocytic leukemia (CMML). These updated results were featured in an oral presentation at the 2024 American Society of Hematology (ASH) Annual Meeting in San Diego, California.
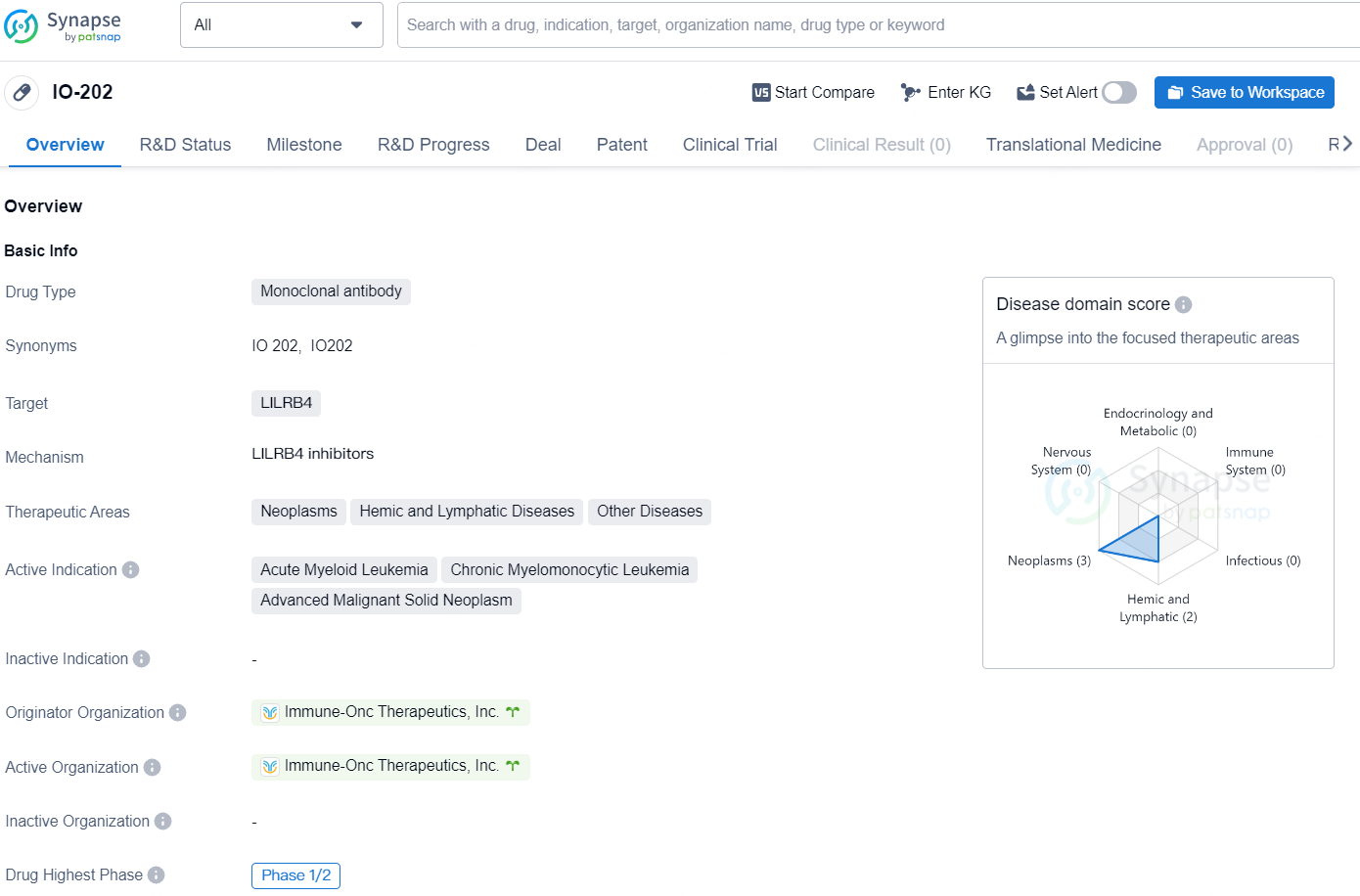
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The latest trial outcomes for hypomethylating agent-naïve CMML patients indicate that the combination of IO-202 and AZA achieved a complete remission (CR) rate of 50.0% and an overall response rate (ORR) of 66.7% among 18 patients assessed for efficacy, according to the International Working Group (IWG) 2023 criteria for higher-risk myelodysplastic syndromes. Further analysis showed that patients with high LILRB4 expression had a CR rate of 83.3% (5/6) and an ORR of 100% (6/6). Responses were noted across all subgroups, including those with proliferative disease, elevated blast counts, unfavorable mutations, and high symptom scores. These results highlight IO-202's potential to meet a significant unmet need in CMML, where the only approved hypomethylating agent treatment provides modest CR rates of 7-17%.
There is an urgent need for innovative CMML treatments, as no new therapies with novel mechanisms have been approved in over three decades. The prognosis for CMML patients is grim, with a median survival of under three years. Allogeneic hematopoietic cell transplant (HCT) is the only potentially curative option, yet less than 15-20% of patients are eligible for HCT. As a novel agent, IO-202 shows promising clinical benefits, addressing a substantial unmet need.
In this study, IO-202 combined with AZA allowed 38.9% of patients to successfully proceed to HCT. Moreover, four out of six transfusion-dependent patients became transfusion-independent. IO-202 was well-tolerated at the preliminary recommended Phase 2 dose (60 mg/kg followed by 30 mg/kg every two weeks) among 21 safety-evaluable patients, with no dose-limiting toxicities reported.
"These findings mark a significant step forward in CMML treatment, a condition with few options and poor outcomes," said Gabriel N. Mannis, M.D., IO-202 Phase 1 investigator and associate professor of medicine, division of hematology, Stanford Cancer Institute, Stanford University. "The positive correlation between LILRB4 expression and treatment response highlights IO-202's contribution to efficacy, aligning with its mechanism of action. Achieving a 50% overall complete response rate—and an 83% complete response rate in patients with high LILRB4 expression—suggests IO-202's potential as a highly effective therapy. Furthermore, the ability to facilitate nearly 40% of patients to transplant and assist most transfusion-dependent patients in achieving independence offers new hope for improving outcomes in this challenging disease.”
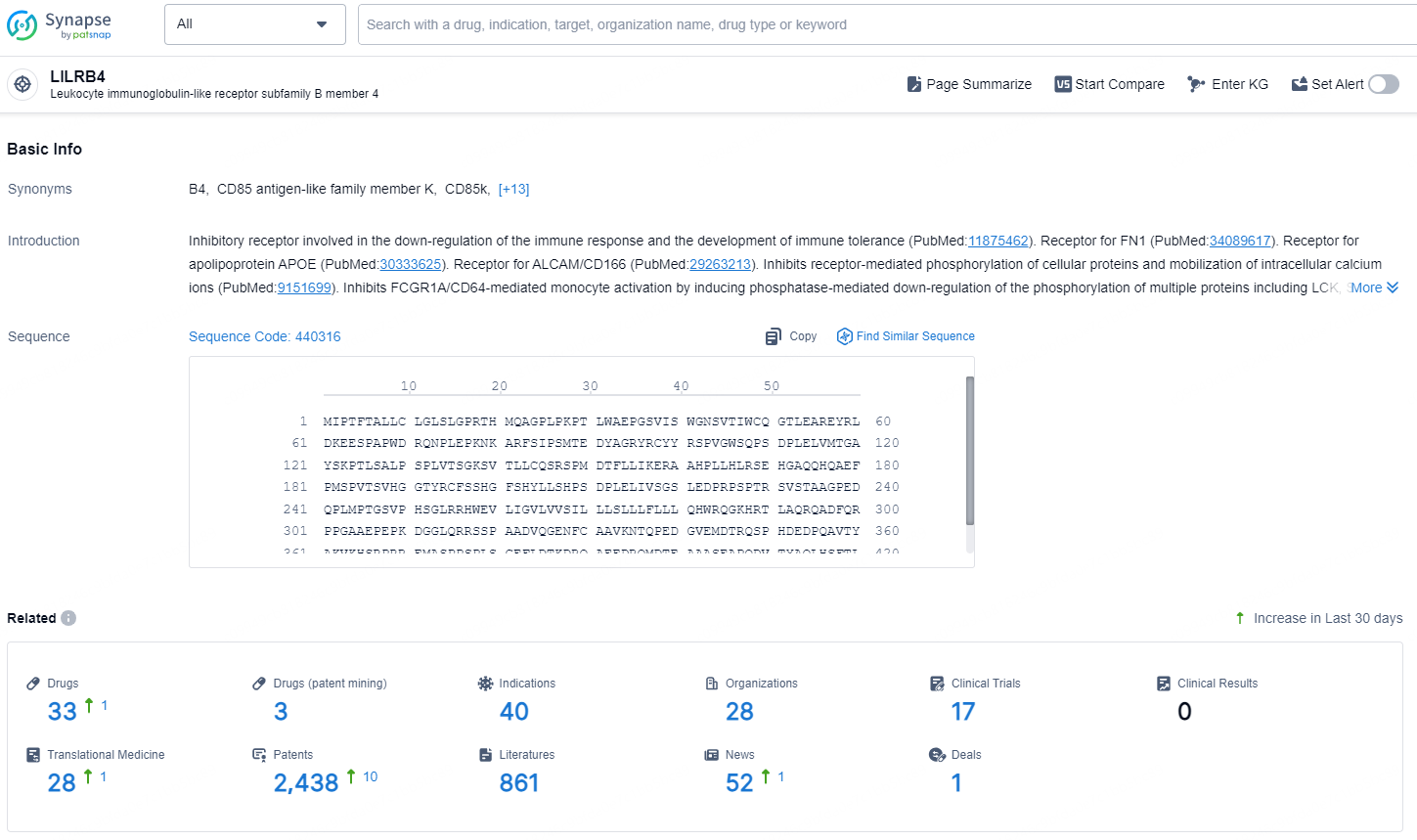
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 13, 2024, there are 33 investigational drug for the LILRB4 target, including 40 indications, 28 R&D institutions involved, with related clinical trials reaching 17,and as many as 4381 patents.
IO-202 is a monoclonal antibody drug developed by Immune-Onc Therapeutics, Inc. The drug targets LILRB4 and is being developed for the treatment of neoplasms, hemic and lymphatic diseases, and other diseases. Its active indications include acute myeloid leukemia, chronic myelomonocytic leukemia, and advanced malignant solid neoplasm.