Immutep Wins Approval for Phase I Trial of New LAG-3 Agonist for Autoimmune Diseases
Immutep Limited, a biotechnology firm at the clinical stage focusing on innovative LAG-3 immunotherapies for cancer and autoimmune conditions, reports it has secured approval from both the ethical committee and the regulatory authorities in the Netherlands to commence the initial Phase I trial of IMP761 in humans.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
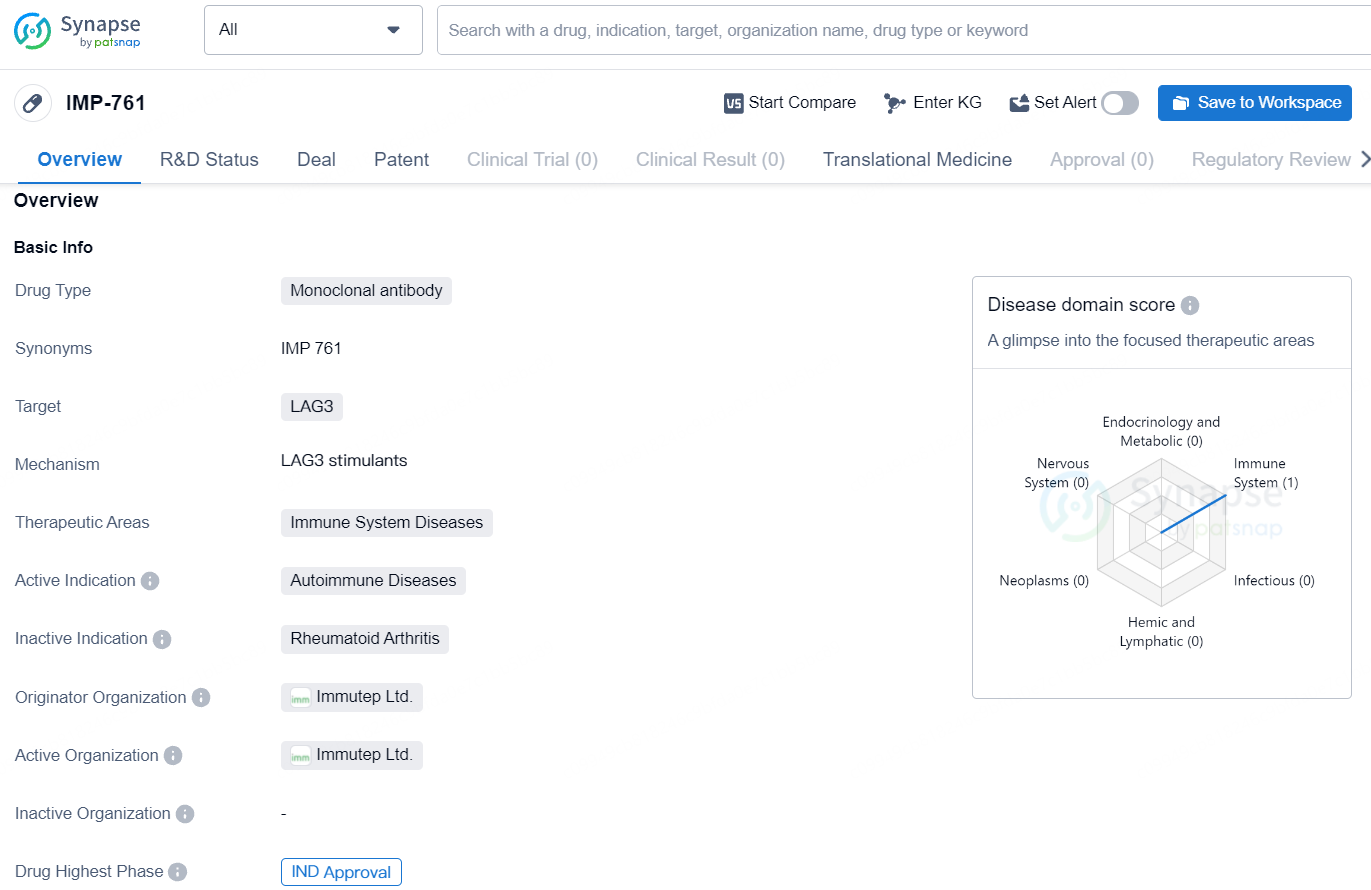
IMP761 is recognized as the first-ever therapeutic LAG-3 agonist antibody, placing it at a unique advantage in the landscape of autoimmune disease treatment. The immune checkpoint molecule LAG-3 has been identified as a valuable target for agonist-based immunotherapy aimed at conditions like rheumatoid arthritis, Type 1 diabetes, and multiple sclerosis, among other autoimmune disorders. IMP761’s mechanism involves enhancing the “brake” function of LAG-3, thereby restoring immune system balance by suppressing the activity of unregulated self-antigen-specific memory T cells. These memory T cells aggregate at sites of disease and are implicated in the pathogenesis of various autoimmune conditions.
According to Professor Bent Deleuran, MD, from Aarhus University’s Department of Biomedicine, "Immune checkpoint molecules such as LAG-3 are crucial in regulating the outcomes of antigen activation and thus hold great therapeutic promise for autoimmune diseases. It is very encouraging to see IMP761 advancing to clinical trials to assess its potential as a novel immunotherapy for autoimmune disorders."
As a pioneering LAG-3 agonist antibody with immunosuppressive properties, IMP761 aims to directly address the fundamental mechanisms of autoimmune diseases by targeting and silencing autoimmune memory T cells, which tend to accumulate in disease-affected areas, and by rebalancing the immune response. Published data in the Journal of Immunology reveals that IMP761 effectively suppresses peptide-induced T cell proliferation, the activation of primary human T cells, and antigen-specific delayed-type hypersensitivity reactions in encouraging pre-clinical in vivo and in vitro experiments.
Furthermore, preclinical findings in oligoarticular juvenile idiopathic arthritis, presented in Pediatric Research, demonstrate that IMP761 can significantly reduce a wide range of effector cytokines within 48 hours. The study also noted that children with o-JIA exhibit altered LAG-3 metabolism, suggesting they could benefit from the agonistic activity of LAG-3.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
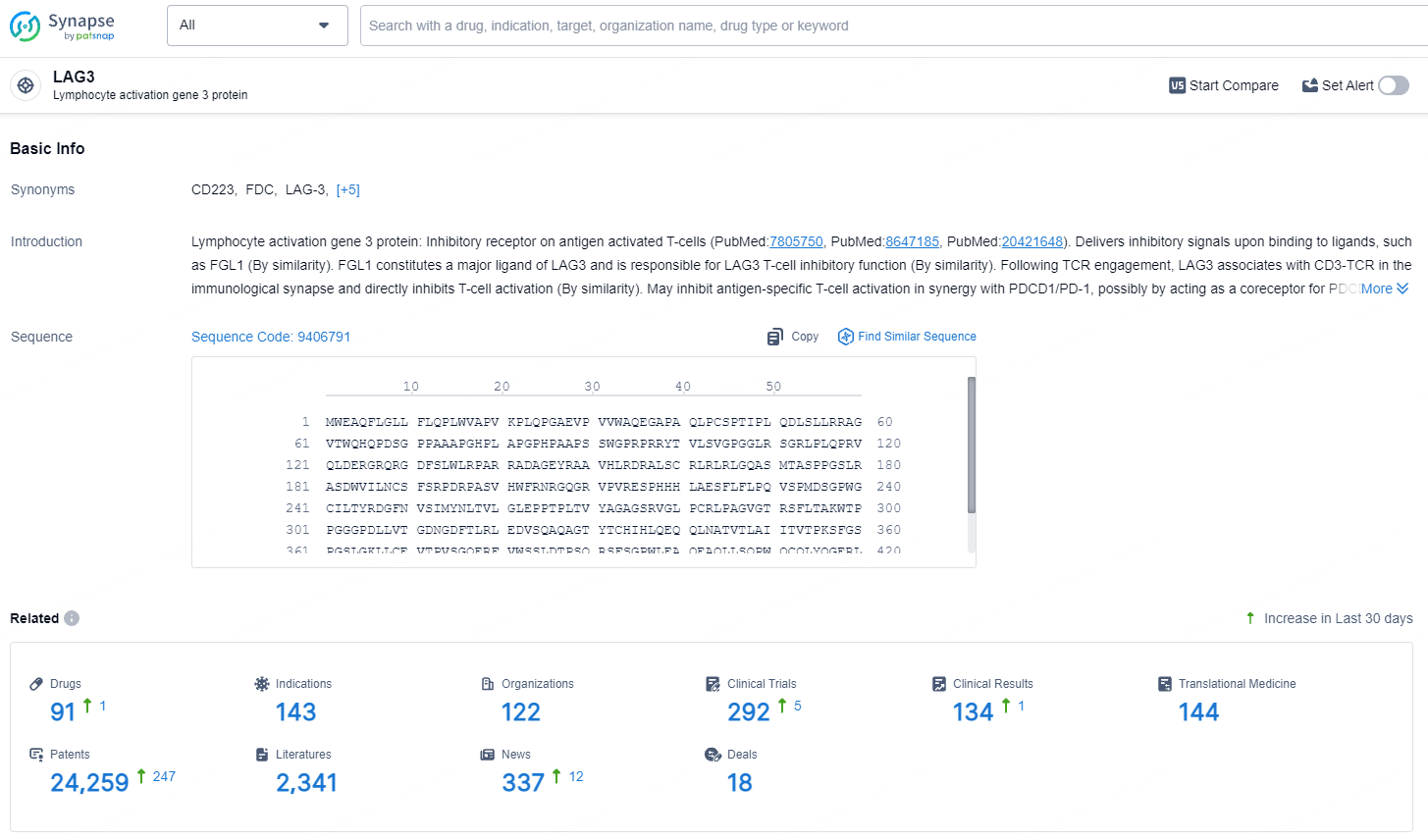
According to the data provided by the Synapse Database, As of July 23, 2024, there are 91 investigational drugs for the LAG-3 target, including 143 indications, 122 R&D institutions involved, with related clinical trials reaching 292, and as many as 24259 patents.
IMP-761 is a monoclonal antibody drug developed by Immutep Ltd. that targets LAG3 and is intended for the treatment of autoimmune diseases. With the approval of an IND application, the drug has shown promise in its development and may offer a targeted and potentially effective treatment option for patients with immune system disorders.