FDA Grants Priority Review for Atara's Tab-cel® in Epstein-Barr Virus-Related Disease
Atara Biotherapeutics, Inc., a pioneer in T-cell immunotherapy, utilizes its innovative allogeneic Epstein-Barr virus (EBV) T-cell platform to create groundbreaking treatments for cancer and autoimmune disease patients. Recently, the company announced that the U.S. Food and Drug Administration has accepted its Biologics License Application for tabelecleucel (tab-cel). This application proposes the use of tabelecleucel as a monotherapy for adult and pediatric patients aged two years and older with Epstein-Barr virus positive post-transplant lymphoproliferative disease, who have undergone at least one previous treatment.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 For individuals undergoing solid organ transplants, previous treatment typically includes chemotherapy unless deemed unsuitable. Notably, there are no therapies approved by the FDA for this specific treatment scenario. The Biologics License Application (BLA) has received a Priority Review designation, with a Prescription Drug User Fee Act (PDUFA) target date set for January 15, 2025.
For individuals undergoing solid organ transplants, previous treatment typically includes chemotherapy unless deemed unsuitable. Notably, there are no therapies approved by the FDA for this specific treatment scenario. The Biologics License Application (BLA) has received a Priority Review designation, with a Prescription Drug User Fee Act (PDUFA) target date set for January 15, 2025.
“The approval of the tab-cel BLA marks a key step towards providing this pioneering treatment to patients within the U.S.,” remarked Pascal Touchon, President, and CEO of Atara. “The FDA’s priority review highlights the critical unmet need in EBV+ PTLD, a severe condition with few treatment options and low survival rates. We are diligently collaborating with Pierre Fabre Laboratories to prepare for a potential U.S. launch in early 2025, alongside potential label expansion in the multicohort Phase 2 EBVision trial.”
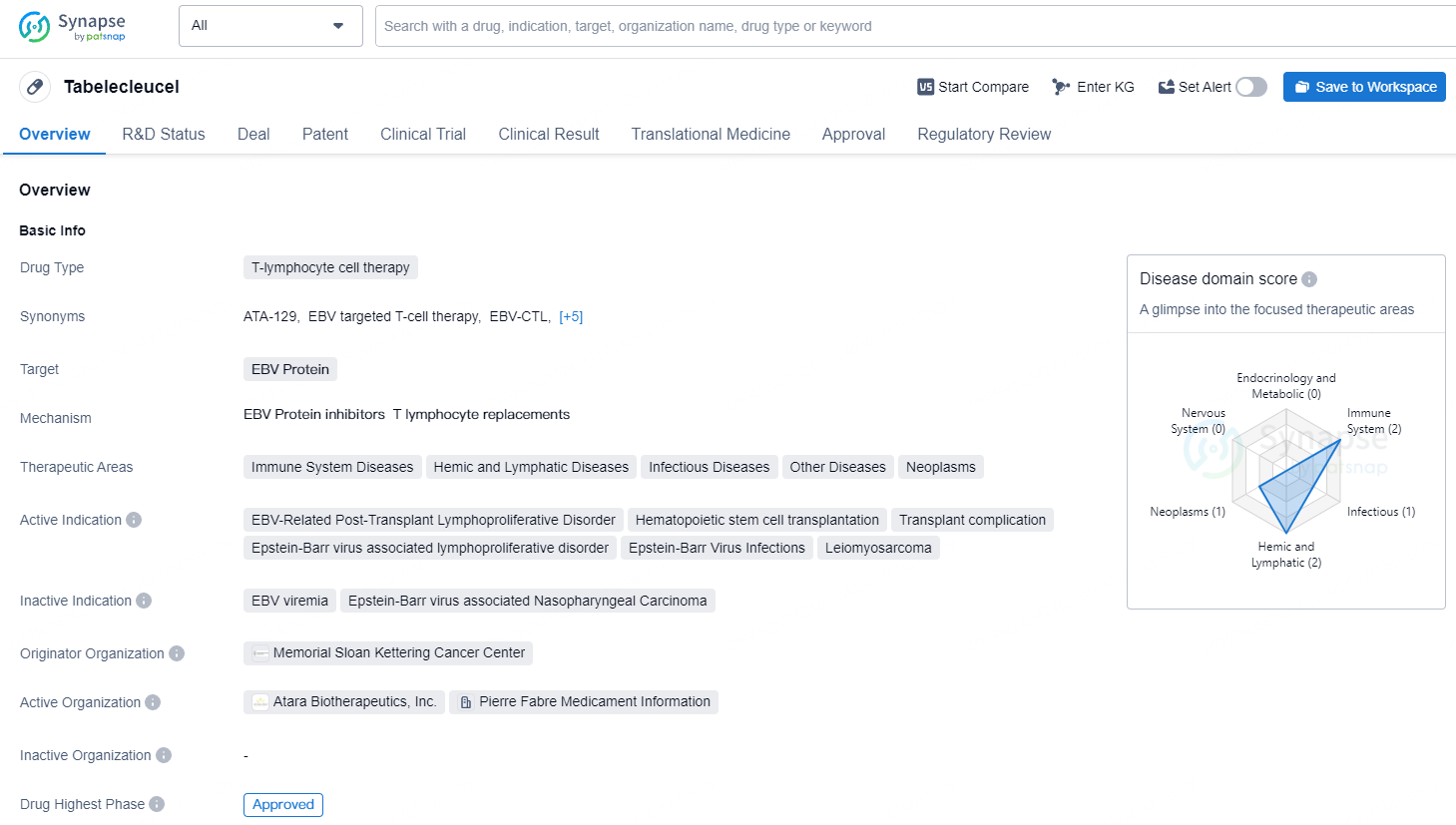
Tab-cel is an allogeneic, EBV-specific T-cell immunotherapy aimed at targeting and destroying cells infected by EBV. The BLA is backed by pivotal and supportive clinical data involving over 430 patients treated with tab-cel for various life-threatening conditions, including recent pivotal ALLELE study data showing a significant 48.8% Objective Response Rate and a safety profile consistent with prior analyses.
The U.S. FDA has given tab-cel a Breakthrough Therapy Designation for treating rituximab-refractory EBV-associated lymphoproliferative disease, along with orphan drug status.
The European Commission granted marketing authorization under the brand Ebvallo™ in December 2022. Ebvallo also received marketing authorization from the Medicines and Healthcare Products Regulatory Agency in the UK in May 2023 and from Swissmedic in Switzerland in May 2024.
In all three regions, Ebvallo is approved as monotherapy for adult and pediatric patients aged two years and older with relapsed or refractory EBV+ PTLD who have undergone at least one prior treatment. For solid organ transplant recipients, the prior treatment generally includes chemotherapy, unless it is inapplicable. Ebvallo was honored with the 2024 Prix Galien International Award for “Best Product for Orphan/Rare Diseases.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
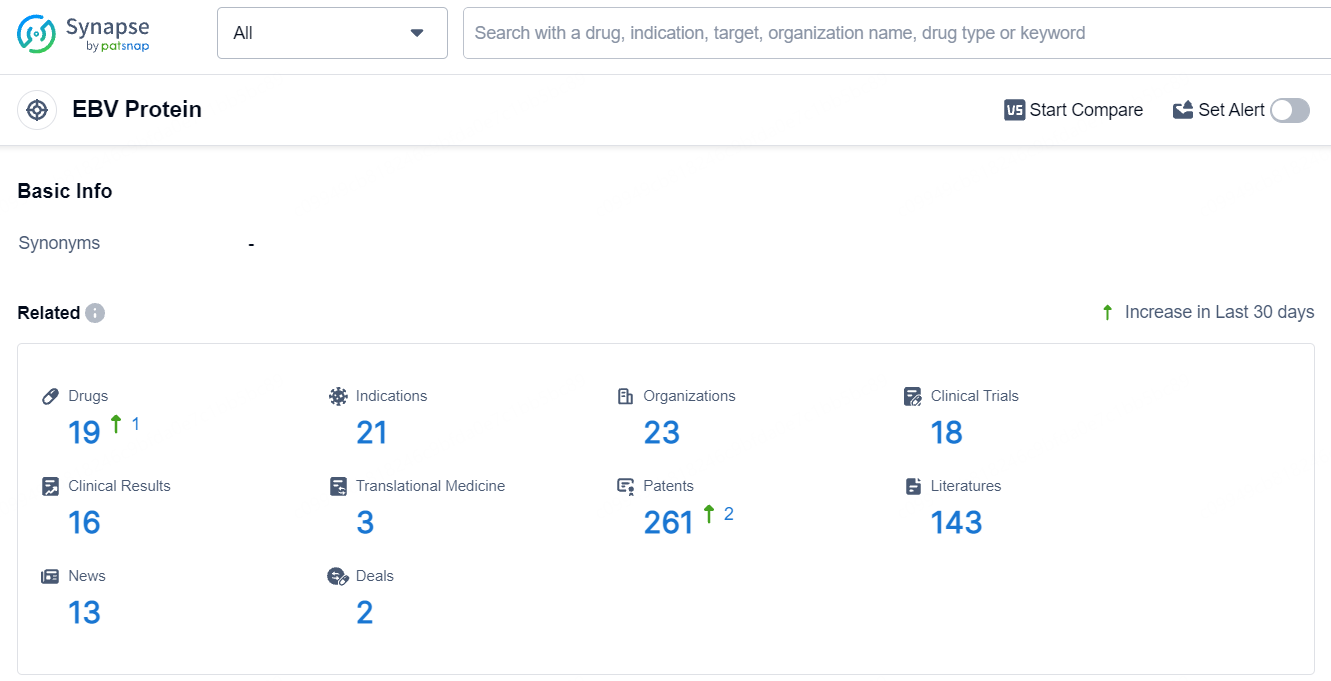
According to the data provided by the Synapse Database, As of July 23, 2024, there are 19 investigational drugs for the EBV target, including 21 indications, 23 R&D institutions involved, with related clinical trials reaching 18, and as many as 261 patents.
Tabelecleucel is a T-lymphocyte cell therapy drug that targets the EBV protein for the treatment of various diseases and conditions related to EBV. Its approval in multiple European countries in 2022 and the various regulatory designations it has received signify its potential as an innovative and impactful drug in the biomedicine industry.