Important anti-cancer drug for tackling tumors: saRNA drug
Research has found that the selective disruption of gene promoter regions with 21-nt Small dsRNAs can activate gene transcription. These Small dsRNAs are known as saRNA (Small activating RNA) and RNA activation offers a new direction for gene therapy.
Due to the small molecular weight and gene specificity of saRNA, it can be used to enhance the expression of specific proteins (products of tumor suppressor gene or haploinsufficient genes). dsP21-322 is a type of saRNA that increases p21 expression and has shown to inhibit tumor growth in preclinical trials of tumor models (prostate cancer, lung cancer, pancreatic cancer). Local delivery of p21 saRNA liposomes also demonstrated anti-cancer effects in bladder cancer and colorectal cancer models. The saRNA of p53 also showed similar anti-tumor effects. It should be noted that in these xenograft models, an intact immune system function is often missing, and most are intratumoral deliveries, which do not reflect the impact of the normal immune system on saRNA.
Similar to other RNA drugs (antisense oligonucleotides/ASO, RNAi, siRNA etc.), saRNA works by binding to target nucleic acids, but unlike others, saRNA ultimately activates gene expression.
saRNA works by relying on the complementarity of antisense or guide strand oligomers to the expected target DNA 5' area, which can be proven by pull-down and ChIP experiments. The position of the 2nd to 8th nucleotides at the 5' end of the guide strand determines the insertion area of saRNA, and mutations in this area affect the activity of saRNA.
Upon internalization, saRNA is recognized by dsRNA loading factors in the cytoplasm, and is preferentially loaded into AGO2 protein. AGO2 does not induce cleavage of RNA targets complementary to saRNA, suggesting that the "scissor activity" of AGO2 is insensitive to saRNA activity. When interacting with saRNA, AGO2 releases one of the two dsRNA strands, i.e., the passenger strand, and the retained strand is called the guide strand or antisense sequence. By design, and modifying the 5' end of the passenger strand, the chances of AGO2 loading are reduced to enhance the retention of AGO2.
Some heterogeneous nuclear ribonucleoproteins, especially hnRNPA2/B1 are required for saRNA action. A complex composed of guide RNA, hnRNPs, and AGO2 is imported into the cell nucleus. It directly binds with DNA in the nucleus, promoting the assembly of the RNA-Induced Transcriptional Activation (RITA) complex.
RITA consists of RNA helicase A (RHA), RNA polymerase associated protein CTR9 homolog (part of PAF1 complex), and DEAD-box helicase 5 (DDX5). This complex interacts with RNA polymerase II (RNAPII) to initiate transcription and ensure mRNA elongation. This process involves the relocation of nucleosomes and the formation of nucleosome depletion areas at genome locations, such as TATA box, CpG islands, proximal enhancers, and proximal promoters. These regulatory binding sites are exposed to facilitate the binding of RNAPII with the transcription start site, and to promote the assembly of the pre-initiation complex.
At the chromatin level, saRNA activation can lead to a decrease in acetylation of histone H3K9 and H3K14, an increase in di/tri-methylation of histone H3K4, a decrease in di-methylation of H3K9, an increase in di-methylation of H3K4, and an increase in mono-ubiquitination of H2B. The PAF1 complex plays a crucial role in initiating these histone modifications by recruiting key histone modifying factors to the RNAPII complex. In some cases, saRNA may not bind with complementary DNA sequences, but may increase target transcripts and favor epigenetic changes in the promoter region by binding with nascent transcripts associated with promoters or long non-coding RNAs.
In general, compared with traditional mRNA vaccines, the mRNA sequence delivered by saRNA vaccines includes a replicable sequence. After entering the cell, it can self-replicate like a virus by utilizing the host cell, thereby achieving high antigen expression at extremely low doses of injection, resulting in the ability to produce a strong immune response and inhibit tumor growth.
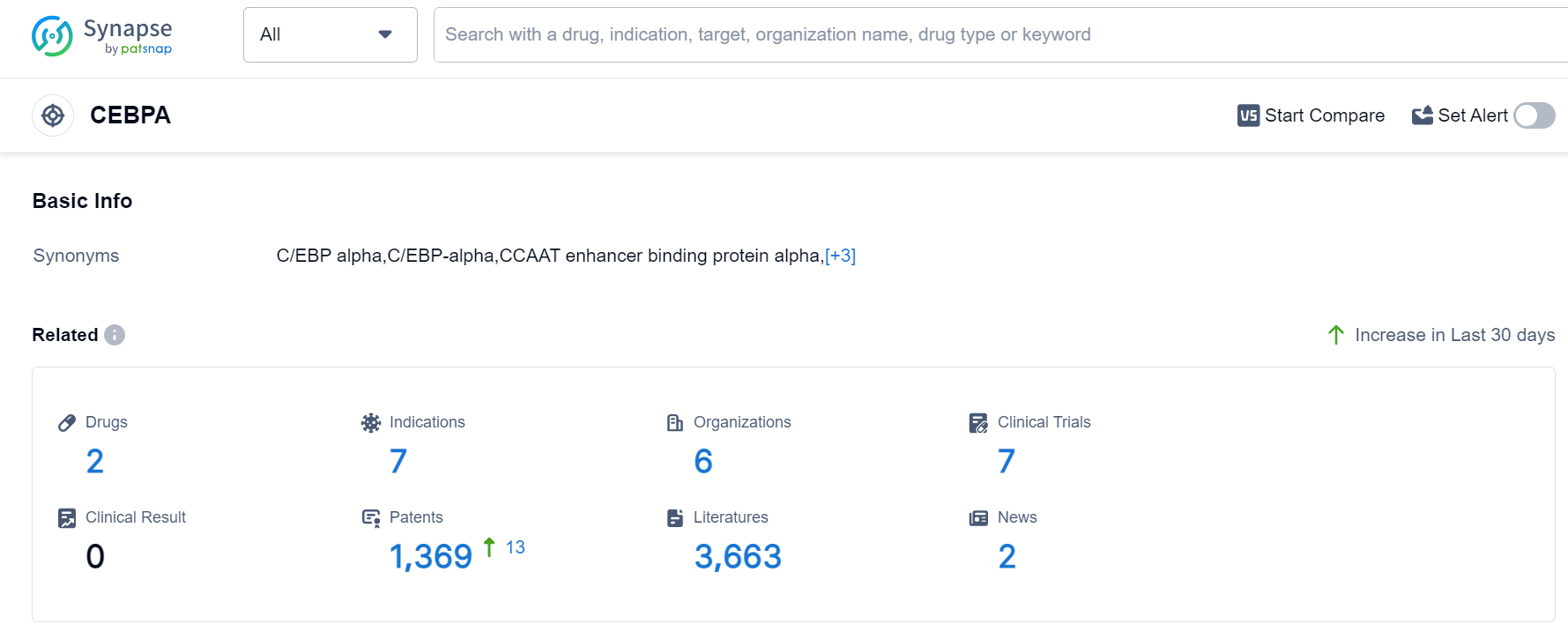
CEBPA Competitive Landscape
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 2 CEBPA drugs worldwide, from 6 organizations, covering 7 indications, and conducting 7 clinical trials.
Drugs targeting CEBPA have been approved for indications such as Hepatocellular Carcinoma, Hepatitis B, Hepatitis C, Idiopathic Pulmonary Fibrosis, Liver Cancer, Colorectal Cancer, and Liver Diseases.
The analysis of the current competitive landscape and future development of target CEBPA.,Mina Alpha Limited, Xfibra, Inc., MiNA Therapeutics Ltd., University of California, The University of Texas at Austin, and Adhera Therapeutics, Inc. are the companies growing fastest under the current target. Singapore, United States, United Kingdom, China are the countries/locations developing fastest, with China showing progress in Phase 1 development. Overall, the analysis suggests a promising future for the development of drugs targeting CEBPA, with ongoing research and development efforts in various companies, indications, drug types, and countries/locations.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Phase II Clinical Trial of saRNA Medicinal: MTL-CEBPA
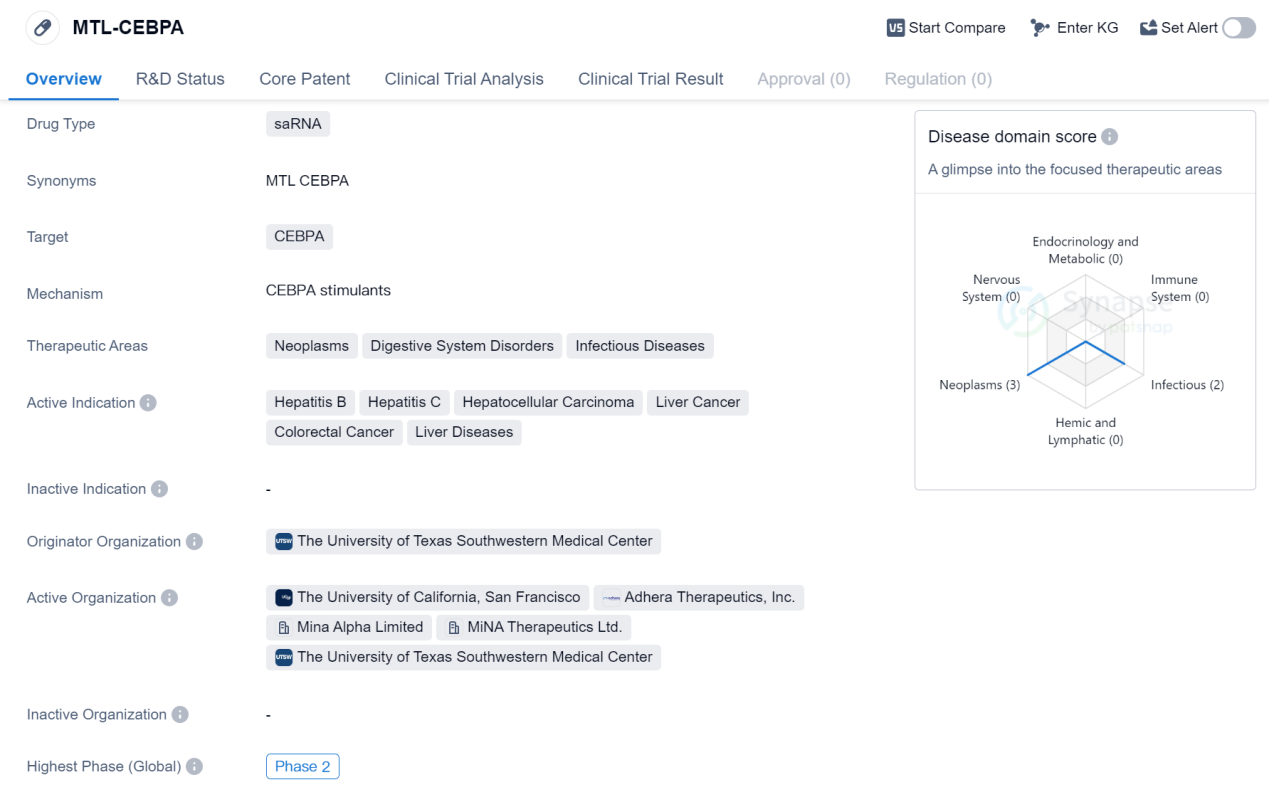
MTL-CEBPA is a saRNA drug that targets CEBPA, a protein involved in various diseases. It has shown potential therapeutic benefits in the treatment of neoplasms, digestive system disorders, and infectious diseases. The active indications for MTL-CEBPA include hepatitis B, hepatitis C, hepatocellular carcinoma (liver cancer), colorectal cancer, and liver diseases.
The drug is being developed by The University of Texas Southwestern Medical Center, which serves as the originator organization. Currently, MTL-CEBPA has reached Phase 2 of clinical trials globally, indicating that it has already undergone extensive testing in humans. In China, the drug has progressed to Phase 1, suggesting that it is in the early stages of clinical development in this region.
As a saRNA drug, MTL-CEBPA utilizes small activating RNA molecules to target and modulate the activity of CEBPA. This approach holds promise in the field of biomedicine as it allows for precise and specific targeting of disease-related proteins. By targeting CEBPA, MTL-CEBPA aims to address the underlying mechanisms of various diseases, including those affecting the liver and digestive system.
The therapeutic areas of neoplasms, digestive system disorders, and infectious diseases encompass a wide range of conditions, highlighting the potential versatility of MTL-CEBPA. Hepatitis B and hepatitis C are viral infections that primarily affect the liver, while hepatocellular carcinoma and colorectal cancer are forms of cancer that can have devastating effects on patients. Liver diseases, which can include conditions such as cirrhosis and fatty liver disease, also fall within the scope of MTL-CEBPA's active indications.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Overall, MTL-CEBPA shows promise as a saRNA drug targeting CEBPA for the treatment of various diseases. Its advancement to Phase 2 globally and Phase 1 in China indicates that it has demonstrated positive results in earlier stages of clinical development. Further research and clinical trials will be necessary to fully evaluate the safety and efficacy of MTL-CEBPA in treating neoplasms, digestive system disorders, and infectious diseases, particularly those affecting the liver.