Improvement in Asthma Patients One Year After Mepolizumab Treatment
Data analysis from a sample of 822 patients reveals noticeable mepolizumab in symptomatology among Mepolizumab-treated adult asthma sufferers a year post-treatment, regardless of the existence of concomitant medical conditions.
The study conducted by Dr. Mark C. Liu from Johns Hopkins University, Baltimore and his professional associates, underscores that comorbidity conditions such as chronic rhinosinusitis with nasal polyposis, gastro-oesophageal reflux disorder, emotional disorders including anxiety and depression, in addition to chronic obstructive pulmonary disorder (COPD), are frequently observed in patients with severe asthma, thereby augmenting the strain of the disease
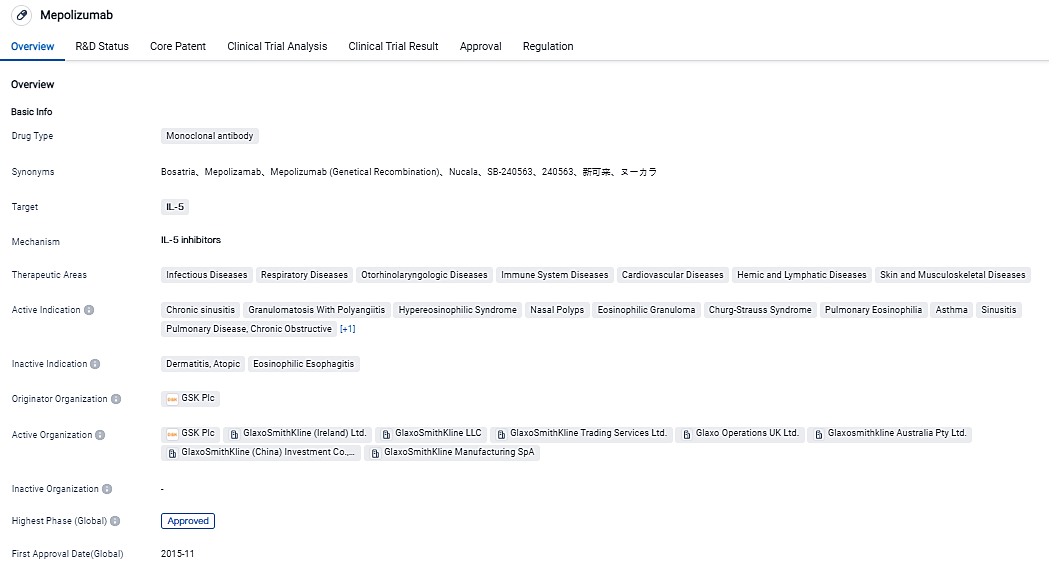
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The researchers stated that certain comorbidities like CRSwNP have overlapping pathophysiological aspects with serious asthma, which involve interleukin-5(IL-5), and that targeting IL-5 for treatment could enhance results. In the practical application via the REALITI-A research, mepolizumab - a humanized monoclonal antibody aimed at IL-5, managed to notably decrease asthma-related flare-ups and usage of oral corticosteroids in severe asthma sufferers.
In order to gauge the influence of mepolizumab on patients with comorbidities, the research team carried out a post hoc analysis which involved 822 grownups who suffer from severe asthma, counting among them 321 with CRSwNP, 309 with GERD, 203 with depression/anxiety and 81 with COPD. The CSE rates across all the various comorbidities dropped considerably from the phase prior to treatment to the consecutive phase, moving from 4.28 incidents yearly to 1.23 incidents yearly
An increased numerical reduction in CSE rates was observed in patients diagnosed with CRSwNP as opposed to those without, whereas the opposite was seen in patients diagnosed with COPD and depression/anxiety. Still, the confidence intervals for the COPD subgroup were quite extensive, said the researchers.
Post mepolizumab treatment, the median maintenance dosage of oral corticosteroids was reduced by a minimum of 50% across all comorbidities with patients diagnosed with CRSwNP showing the largest decline (83%).Furthermore, the Asthma Control Questionnaire–5 scores went down by at least 0.63 points, and the forced expiratory volume per second (FEV1) improved from the baseline across all comorbidities by a minimum of 74 mL post mepolizumab treatment.
The researchers mentioned several limitations to these findings including volunteer assessments having incomplete data, the post hoc design, fairly small patient numbers in certain subgroups especially COPD, and potentially incorrect COPD diagnoses.
But, since the improvements in each outcome post mepolizumab treatment varied based on the comorbidity, the study's findings underscore the influence of comorbidities along with their occurrence and severity on outcomes.
The overall accomplishment of mepolizumab across clinical characteristics and comorbidities backs the general applicability of these findings to the wider adult population suffering from serious asthma, the researchers concluded.
The study had financial backing from GlaxoSmithKline. Dr. Liu made disclosures about obtaining research funding from GSK, Boehringer Ingelheim, and Gossamer Bio, in addition to being part of advisory panels for AstraZeneca, GSK, and Gossamer Bio.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
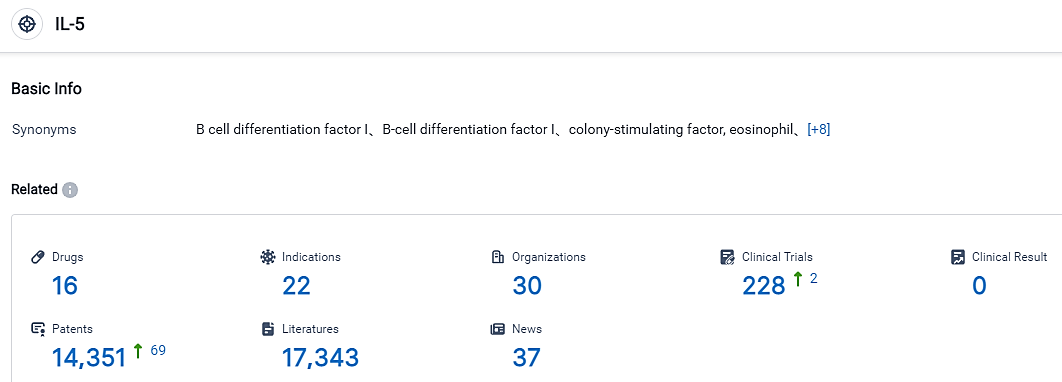
According to the data provided by the Synapse Database, As of September 3, 2023, there are 16 investigational drugs for the IL-5 target, including 22 applicable indications, 30 R&D institutions involved, with related clinical trials reaching 228, and as many as 14351 patents. The count of chronic obstructive pulmonary disease (COPD) cases globally was estimated to be around 317.3 million, with projections indicating a rise to 328.9 million by 2027. Should they receive approval and payer coverage, high-cost biologics could potentially drive market growth as a new inclusion in physicians’ treatment repertoire for more severe COPD patients.
Nevertheless, the usage of IL-5 inhibitors is expected to be confined to COPD patients with raised eosinophil levels, which might comprise about one third of all COPD patients, since they are likeliest to experience benefits.