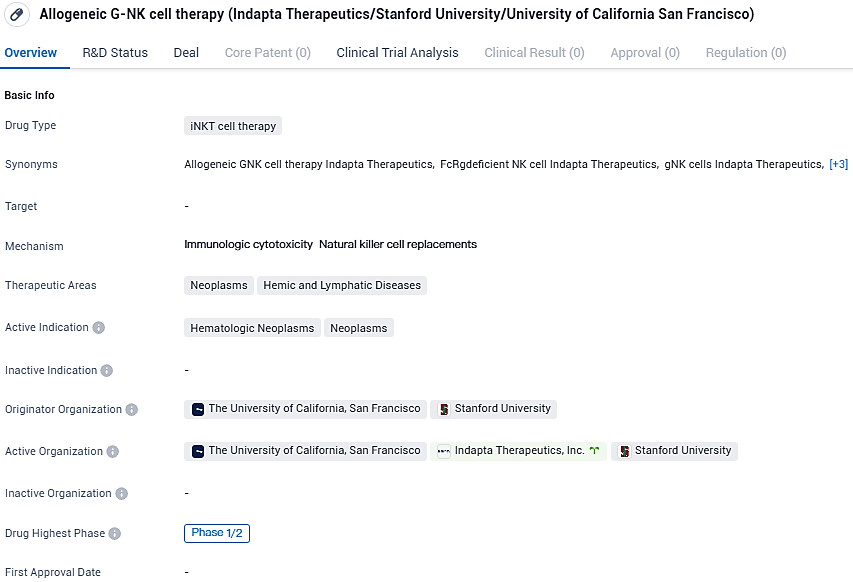
Indapta Therapeutics Begins Treating Patients with IDP-023, a Non-Autologous NK Cell Cancer Therapy
Indapta Therapeutics, Inc., an emerging biotech enterprise focused on advancing IDP-023, its proprietary natural killer cell therapeutic for oncology applications, has commenced the administration of this treatment to the inaugural cohort of participants in its Phase 1 clinical study. This study targets individuals diagnosed with multiple myeloma and Non-Hodgkin’s lymphoma. Administration of the therapy has taken place at two reputable institutions: the University of Texas MD Anderson Cancer Center and NEXT Oncology in Virginia, signaling the start of a critical phase of clinical evaluation for Indapta Therapeutics’ innovative cancer therapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The inaugural participant was administered a one-time dosage of IDP-023. A second participant has commenced a regimen involving the initial dose of a series of three injections of IDP-023. Future groups of participants are slated to undergo a trio of IDP-023 administrations, which might be concomitant with or without the incorporation of interleukin-2. After confirming the safety profile of combined multiple dosing with interleukin-2, specific patient groups diagnosed with lymphoma and multiple myeloma are set to be treated with a therapeutic protocol that incorporates IDP-023 in conjunction with targeted monoclonal antibodies—rituximab for the former and daratumumab for the latter.
Dr. Mark Frohlich, the Chief Executive Officer at Indapta, expressed enthusiasm over the company's progression to the clinical phase. "With the promising data regarding NK cell-mediated therapeutic effects from other studies and IDP-023's pronounced efficacy over traditional NK cells in preclinical settings, we are eager to proceed with the assessment of its safety and therapeutic potential during the initial phase of clinical testing," he declared.
Further announcements from Indapta signal that the company has effectively produced an ample quantity of IDP-023 to sustain the needs of the Phase 1 trial until the latter half of 2024. “Our manufacturing yield has met with our high expectations," Dr. Frohlich emphasized. "The recent advancements in our manufacturing procedures, supplemented with upcoming enhancements, should ensure our product stands competitively priced at the time of its introduction to the market."
G-NK cells are distinct immune cells that emerge due to epigenetic alterations linked to cytomegalovirus exposure. Indapta has devised a method to selectively cultivate g-NK cells from a pool of healthy donors, particularly from those exhibiting elevated levels of g-NK cells. The company's innovative off-the-shelf g-NK cell therapeutic product, IDP-023, distinguishes itself from alternative NK cell treatments by virtue of its cryopreserved formulation with reduced variability.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
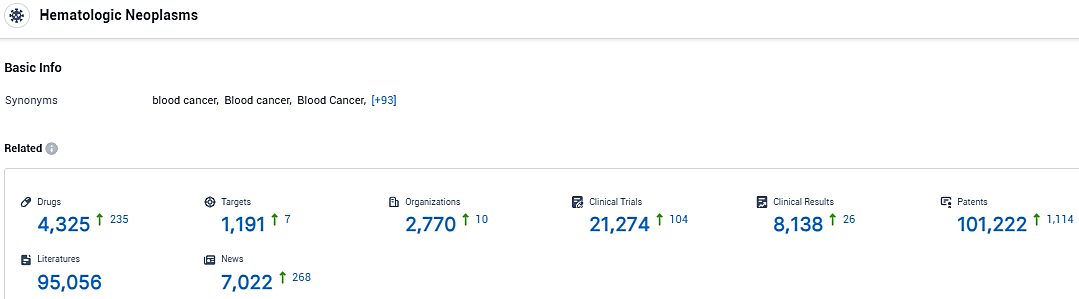
According to the data provided by the Synapse Database, As of January 18, 2024, there are 4325 investigational drugs for hematologic neoplasms, including 1191 targets, 2770 R&D institutions involved, with related clinical trials reaching 21274, and as many as 101222 patents.
IDP-023 shows promise in the field of biomedicine, particularly in the treatment of hematologic neoplasms and neoplasms. As the drug progresses through its clinical trials, further data and analysis will be needed to determine its potential impact on patients and the pharmaceutical industry as.