AskBio, a Bayer subsidiary, initiates Phase II GenePHIT clinical trial for CHF treatment
Bayer AG and Asklepios BioPharmaceutical, Inc., have recently declared the commencement of the GenePHIT trial, which marks the Phase II stage for investigating AB-1002 (NAN-101) as a potential therapy for congestive heart failure. This trial, coined GenePHIT, is designed to be an adaptable study that will be conducted in a double-blind fashion, with a placebo comparison, and will randomly assign participants across multiple centers. Its primary goal is to assess the safety profile and the therapeutic effectiveness of a one-time intracoronary delivery of AB-1002 to patients who have non-ischemic cardiomyopathy, particularly those who fall under the New York Heart Association Class III Heart Failure category. Enrollment is limited to adult individuals who have maintained medical stability for a minimum duration of 4 weeks.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The progression of AB-1002 into its second clinical phase is an important step forward in its development as an experimental gene therapy intended for CHF sufferers who lack adequate medical solutions.
GenePHIT will encompass a cohort of 90 to 150 adult participants with a left ventricular ejection fraction ranging from 15 to 35%, who persistently experience symptoms of heart failure even with established treatment regimens. The definitive measure of effectiveness at 52 weeks will be determined by a weighted collective of several significant clinical evaluations.
“With Roger Hajjar, MD in the role of Scientific Chair for CHF, and Lothar Roessig, MD overseeing the Integrated Product Team for CHF, AskBio is thrilled to move forward with the GenePHIT trial,” announced Jude Samulski, PhD, Co-founder and Chief Scientific Officer at AskBio.
Jude Samulski adds, “Our anticipation is that this study will unveil the therapeutic promise of AB-1002 for a severely impacting global ailment. Our focus is on garnering a deeper understanding of this pioneering cardiac gene therapy area. We are optimistic that AB-1002 may someday deliver relief to those battling with congestive heart failure.”
The GenePHIT study, with scheduled assessments of safety and main efficacy endpoints at one year and extended follow-ups spanning four years, is actively enlisting participants. The intention of AskBio is to manage the study in various European countries as well as within the U.S.
As a product still in its investigative phase, AB-1002 has yet to receive clearance from any health regulatory entities, and its therapeutic effectiveness and safety remain to be completely discerned. Viralgen Vector Core, an autonomous subsidiary fully owned by AskBio, is the manufacturer behind AB-1002.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
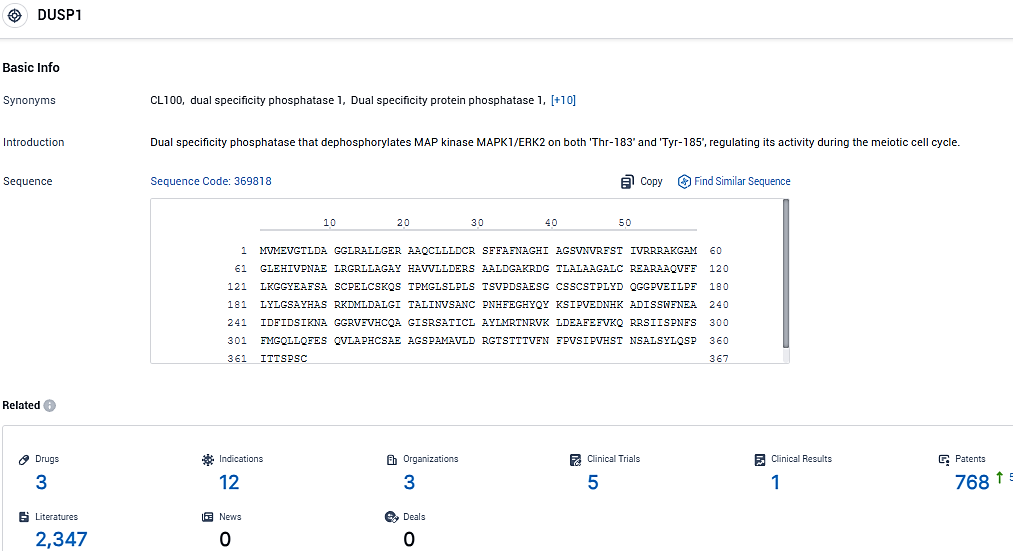
According to the data provided by the Synapse Database, As of January 16, 2024, there are 3 investigational drugs for the DUSP1 target, including 12 indications, 3 R&D institutions involved, with related clinical trials reaching 5, and as many as 768 patents.
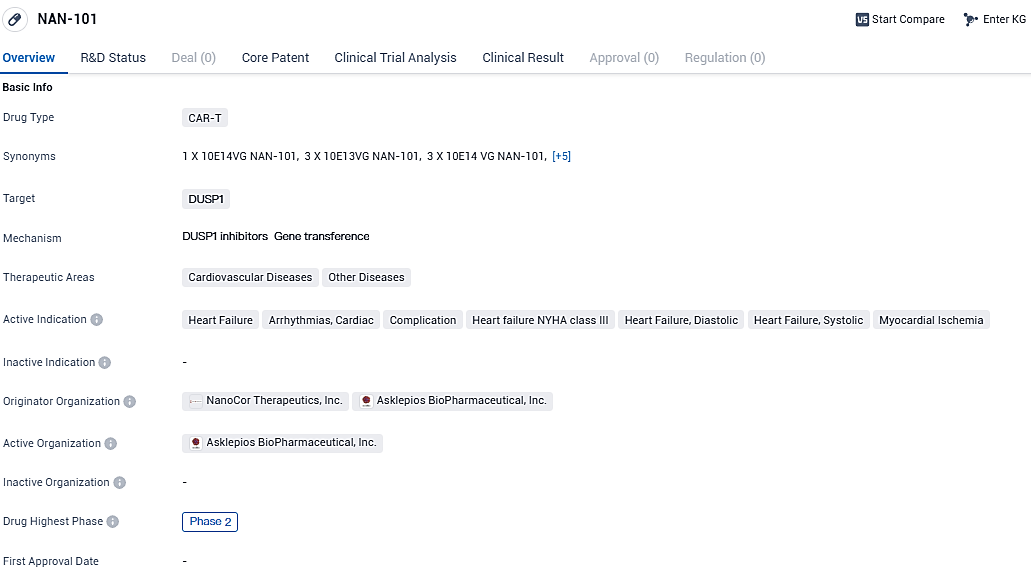
NAN-101 is a CAR-T drug that targets DUSP1 and is being developed for the treatment of cardiovascular diseases. It is currently in Phase 2 of clinical trials and has shown potential in addressing conditions such as heart failure, arrhythmias, and myocardial ischemia. Further research is needed to determine its safety and efficacy in larger patient populations.