Initial Dosing in Phase I Glioblastoma Trial by ITM, Helmholtz Munich, and University Hospital Münster
The Neurosurgery and Nuclear Medicine departments at University Hospital Münster, in collaboration with Helmholtz Munich and ITM Isotope Technologies Munich SE, which is a premier company specializing in radiopharmaceutical biotechnology, have disclosed the commencement of an investigator-initiated trial where the first patient has received a dose of the experimental radiopharmaceutical, ITM-31, aimed at treating glioblastoma. The study, underwritten by the University Hospital Münster, is being performed in medical institutions across Münster, Essen, Cologne, and Würzburg with support from Helmholtz Munich and ITM.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Following standard interventions which include surgical resection, radiation, and chemotherapy, residual glioblastoma cells often persist undetectably within the adjacent tissue, resulting in tumor recurrence. This contributes to glioblastoma’s reputation as an exceptionally aggressive and difficult cancer type with a high mortality rate.
"Emerging from foundational preclinical findings, the phase I trial investigates radiopharmaceuticals as promising agents for overcoming the formidable barriers presented by glioblastoma," stated Prof. Reinhard Zeidler from Helmholtz Munich. Prof. Zeidler played a crucial role in the initial research and oversaw the scientific progression into clinical phases.
Steffen Schuster, CEO of ITM, remarked, “Emerging radiopharmaceuticals reveal significant potential to fill the existing void in treatment strategies, especially for severe and prognostically unfavorable cancers like glioblastoma. ITM is enthusiastic about backing the study led by Prof. Stummer and the team at University Hospital Münster.”
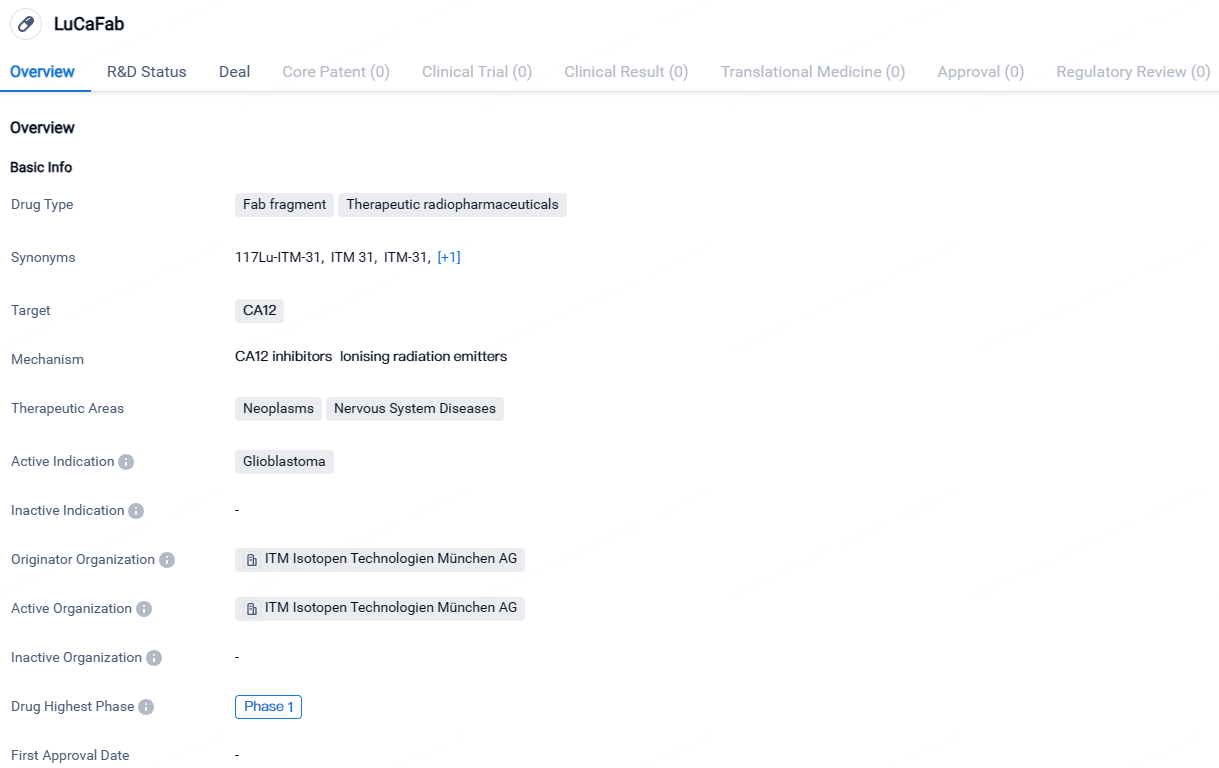
LuCaFab, classified as a therapeutic radiopharmaceutical, is a Fab fragment specifically targeting CA12, a protein linked with various pathological conditions. The primary focus of this drug is on the treatment of neoplasms and neurological conditions, primarily indicated for glioblastoma, a brain cancer type.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
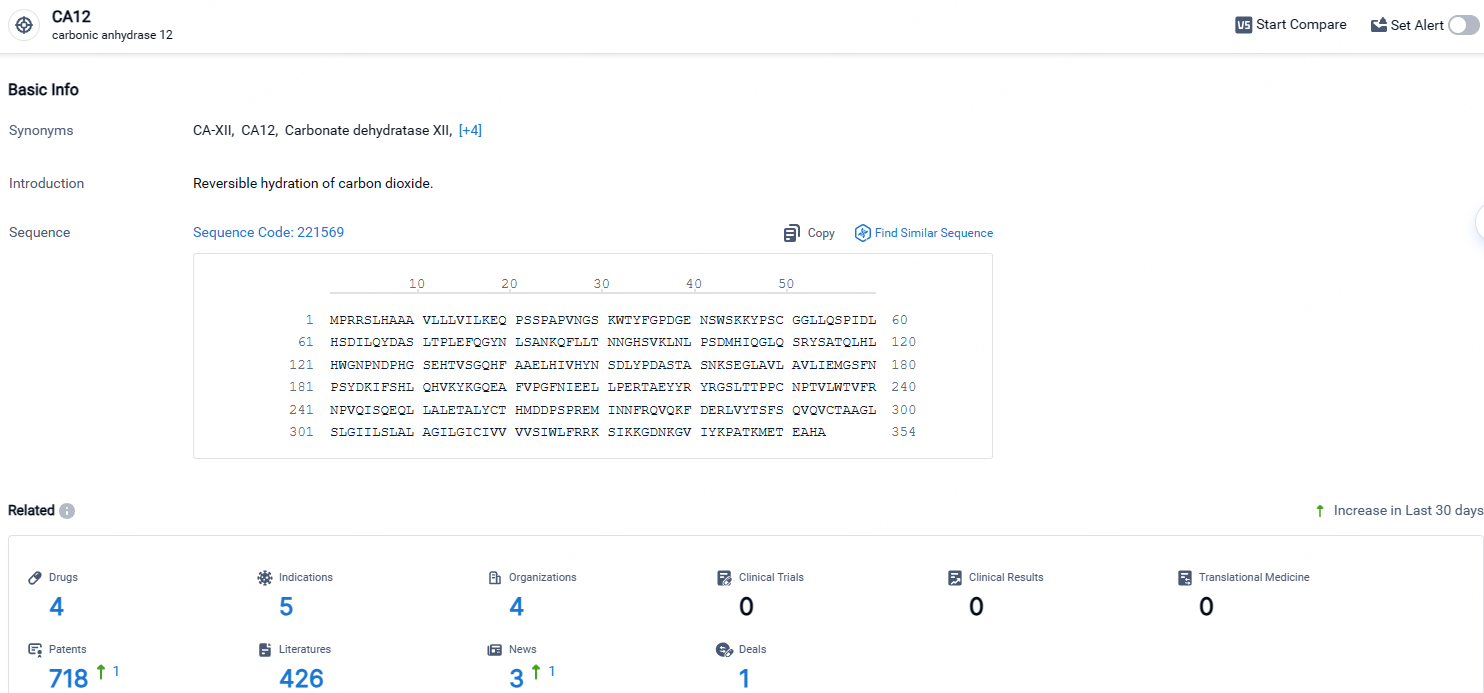
According to the data provided by the Synapse Database, As of April 25, 2024, there are 4 investigational drugs for the CA12 target, including 5 indications, 4 R&D institutions involved, and as many as 718 patents.
The therapeutic areas of neoplasms and nervous system diseases highlight the potential applications of LuCaFab beyond glioblastoma. Neoplasms refer to abnormal growths or tumors, while nervous system diseases encompass a wide range of conditions affecting the brain, spinal cord, and nerves. This suggests that LuCaFab may have the potential to be explored for the treatment of other types of tumors or neurological disorders in the future.