Initial Human Trial of Arvinas's PROTAC® ARV-102 for Treating Neurological Conditions
Arvinas, Inc., which operates in the biotech industry and is currently in the clinical trial phase, has reported the administration of the initial dose in their Phase 1 clinical study for ARV-102. This oral pharmaceutical agent represents the company's innovative venture into PROTAC® protein degradation technology, specifically formulated to address neurological disorders.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
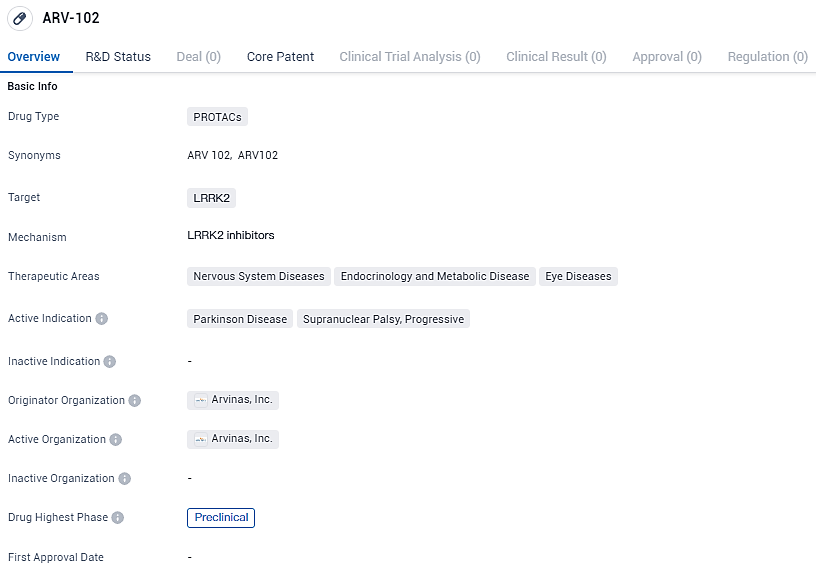
During preliminary research phases, the investigational compound ARV-102 has demonstrated its ability to penetrate the blood-brain barrier and effectively target and reduce the levels of a complex protein called leucine-rich repeat kinase 2 (LRRK2), which plays a pivotal role in the development of certain neurological conditions, such as Parkinson’s disease and progressive supranuclear palsy. Research suggests that an overactive LRRK2 contributes to the etiology of these diseases.
In primate models, ARV-102, when given orally, has successfully reached regions within the central nervous system, considerably reducing LRRK2 presence by up to 90%. Currently, a Phase 1 clinical trial is actively recruiting participants to gauge the compound's safety profile and its effects on the body at the Centre for Human Drug Research located in Leiden, Netherlands. This study aims to assess the safety profile, dose tolerability, movements within the body (pharmacokinetics), and actions on the intended target (pharmacodynamics) of ARV-102. It also seeks to observe the degradation effects on LRRK2 and analyze biomarkers involved in the LRRK2 pathway.
Angela M. Cacace, Ph.D., who serves as the Senior Vice President of Neuroscience and Platform Biology, remarked on the gravity of conditions such as Parkinson’s disease and progressive supranuclear palsy and emphasized the promising development indicated by the administration of ARV-102 to the first healthy volunteer. She noted this as a notable achievement in the journey to produce groundbreaking treatments for those with neurodegenerative conditions.
She further commented on the unique characteristics of ARV-102, emphasizing that it is a novel oral PROTAC (PROteolysis TArgeting Chimeras) degrader specially engineered to not only breach the blood-brain barrier but also specifically dismantle the LRRK2 protein. Given that current therapies primarily aim to inhibit LRRK2 activity, she highlighted that the breaking new ground in targeted protein degradation holds immense potential to revolutionize care strategies for patients with neurodegenerative illnesses.
In a publicized online presentation, both John Houston, Ph.D., who holds the positions of Chairperson, Chief Executive Officer, and President, alongside Angela M. Cacace, Ph.D., shared insights into the ongoing advancements surrounding ARV-102. Additionally, they discussed Arvinas’ continued efforts in expanding their collection of PROTAC protein degraders, specifically targeting neurological diseases. This webcast is accessible through the "Events & Presentations" segment within the "Investors and Media" section on Arvinas’ official website.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
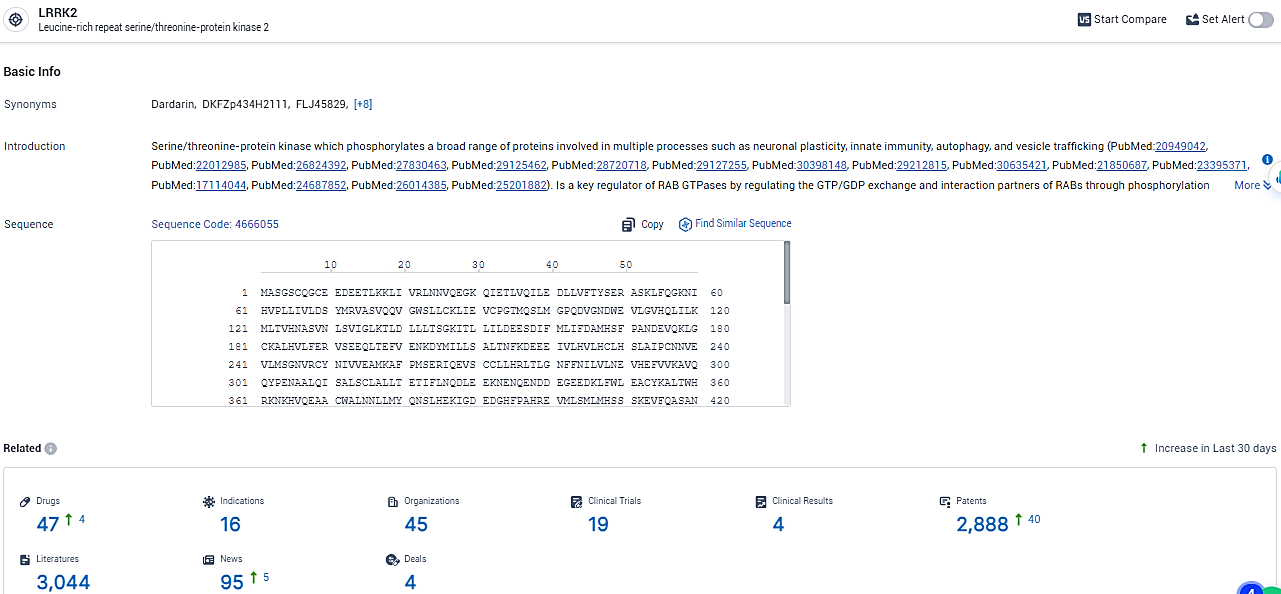
According to the data provided by the Synapse Database, As of February 23, 2024, there are 47 investigational drugs for the LRRK2 target, including 16 indications,45 R&D institutions involved, with related clinical trials reaching 19, and as many as 2888 patents.
ARV-102 targets the protein LRRK2. It has potential applications in treating various nervous system diseases, endocrinology and metabolic diseases, and eye diseases. The active indications for ARV-102 are Parkinson Disease and Supranuclear Palsy, Progressive. Currently, the drug is in the preclinical phase of development, indicating that it is still in the early stages of testing.