Preliminary Phase III Outcomes of Onfekafusp Alfa for Advanced Soft Tissue Sarcoma
Philogen S.p.A. is delighted to confirm the progression of the Phase III FIBROSARC clinical study, in accordance with the established protocol. This determination comes after the Independent Data and Safety Monitoring Board conducted a thorough assessment of the interim analysis data concerning the effectiveness and security of the trial.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The FIBROSARC clinical study is a stage III investigation that randomly allocates participants on a 1:1 basis to assess the impact of combining L19TNF with doxorubicin in comparison to the sole administration of doxorubicin to a group of 118 individuals undergoing initial treatment for advanced or metastatic Soft Tissue Sarcoma.
The primary endpoint for this clinical trial is the duration of Progression-Free Survival, anticipating a substantial 45% diminution in the likelihood of disease progression in the Experimental Arm. The interim analysis, which is an integral part of the study's design, was conducted after achieving half the number of events necessary for determining the primary endpoint.
The threshold of 46 events that constituted the trigger for the interim analysis was achieved on November 9, 2023. At the moment of issuing this press statement, the study has successfully recruited 97 of the 118 participants needed, spanning 24 different medical institutions across Germany, Italy, France, Poland, and Spain. The target is to finalize enrolment of all 118 participants by the year 2024.
Dr. Alfredo Covelli, serving as the Chief Medical Officer at Philogen, made the following statement: "Patients afflicted with advanced or metastatic STS continue to be subjected to chemotherapy protocols that garnered approval back in the 1970s. Despite the entry of cutting-edge treatments, such as immune checkpoint inhibitors, a significant improvement in treatment outcomes for this set of patients has not yet materialized. With anticipation, we await the comprehensive results from the interim evaluation of FIBROSARC, and we have high hopes for what the ultimate data will reveal."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 23, 2024, there are 1 investigational drugs for the EDB-FN and TNF-α target, including 14 indications, 1 R&D institutions involved, with related clinical trials reaching 9, and as many as 157 patents.
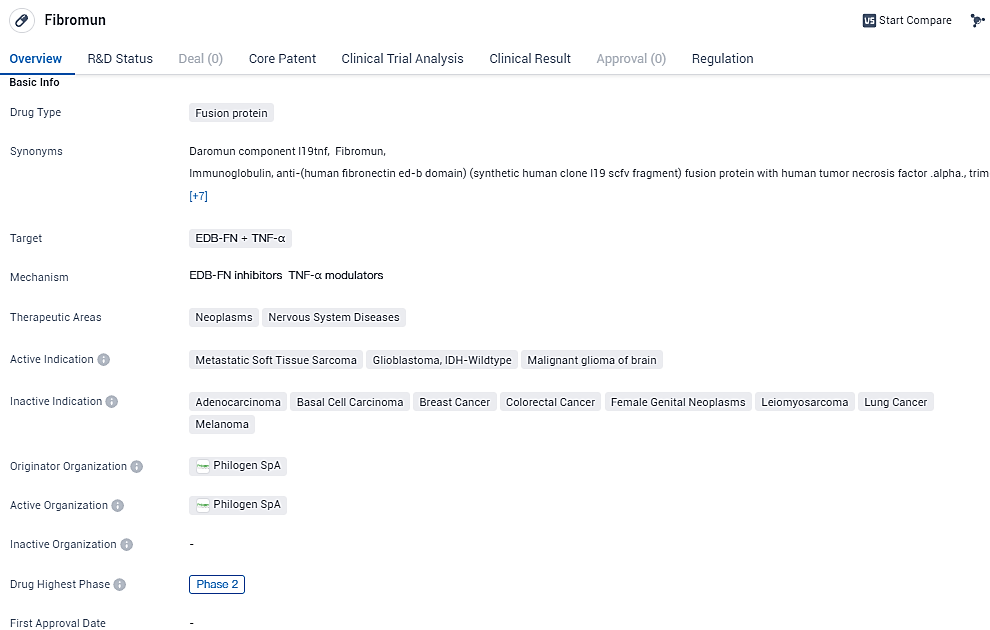
L19TNF is a fusion protein drug being developed for the treatment of neoplasms and nervous system diseases. Fibromun targets EDB-FN and TNF-α. EDB-FN is a protein that is overexpressed in tumor blood vessels, making it a potential target for anti-cancer therapies. With its current status in Phase 2 of development and orphan drug designation, Fibromun shows potential as a targeted therapy for specific indications such as metastatic soft tissue sarcoma and certain types of brain tumors.