Boehringer's Zongertinib Shows Promising Results in Previously Treated HER2-Mutated Lung Cancer
Boehringer Ingelheim announces favorable initial findings from the primary analysis of Cohort 1 in the Beamion LUNG-1 study, which examines zongertinib (BI 1810631) in previously treated patients with advanced non-small cell lung cancer (NSCLC) harboring activating HER2 mutations. In this cohort, zongertinib achieved a significant objective response rate and exhibited a generally acceptable safety profile.
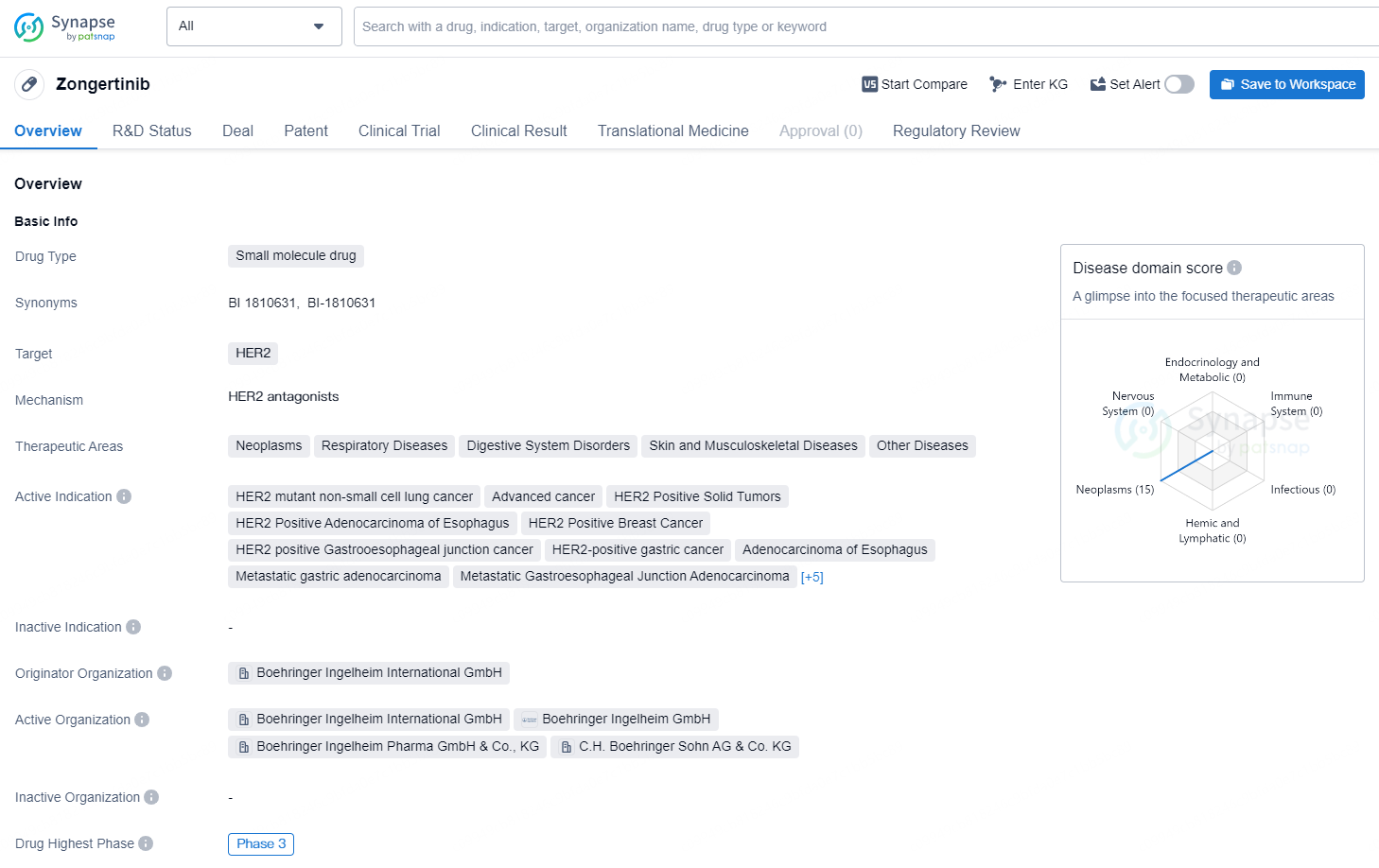
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
By May 2024, a total of 132 patients have received daily doses of zongertinib, either 120 mg (75 patients) or 240 mg (57 patients). The primary endpoint for Cohort 1 (120 mg; 75 patients) was met with an objective response rate (ORR) of 66.7% and a 97.5% confidence interval (CI) of 53.8-77.5%, based on an assessment by a blinded independent central review (BICR) (p<0.0001). Tumor reduction of any degree was seen in 94% of patients across both dosage groups, according to investigator evaluations. The trial included a dose expansion phase aimed at determining the optimal dosage of zongertinib for these patients.
Participants were randomized into two groups: 120 mg (58 patients) and 240 mg (55 patients). Following an interim futility analysis, the 120 mg dose was chosen for further evaluation in Cohort 1, with 17 additional patients enrolled. In the randomized part of the trial, response rates were 72.4% for the 120 mg group and 78.2% for the 240 mg group, while disease control rates (DCRs) were 95% and 100%, respectively.
Data from Phase Ib, Cohort 1 also indicated initial brain activity results with zongertinib. Among patients with asymptomatic brain metastases, 33% (27 patients on 120 mg) and 40% (25 patients on 240 mg) exhibited confirmed objective responses, achieving DCRs of 74% and 92%, respectively, as assessed by RANO-BM standards through BICR. The central nervous system (CNS) is a frequent site for metastasis in NSCLC and is associated with poor prognosis and diminished quality of life. Up to 30% of NSCLC patients with activating HER2 mutations have brain metastases at diagnosis.
Dr. John Heymach, MD, PhD, of The University of Texas MD Anderson Cancer Center, who is the study's principal investigator, remarked, "These new results could signify a turning point in treating non-small cell lung cancer patients harboring activating HER2 mutations. Although these mutations are uncommon, they are pivotal drivers in some cases of non-small cell lung cancer, and current therapeutic options are extremely limited. Patients with this cancer type generally have a poor outlook, with around 50% responding to initial treatment and just 20% to second-line therapy."
Zongertinib is an experimental oral HER2 tyrosine kinase inhibitor (TKI) being developed for patients with advanced NSCLC with activating HER2 mutations. It has been engineered to avoid targeting wild-type EGFR, thereby reducing associated toxicities. The global Phase III Beamion LUNG-2 trial is comparing zongertinib with the standard of care as a first-line treatment in patients with advanced NSCLC harboring activating HER2 mutations, and is currently enrolling participants.
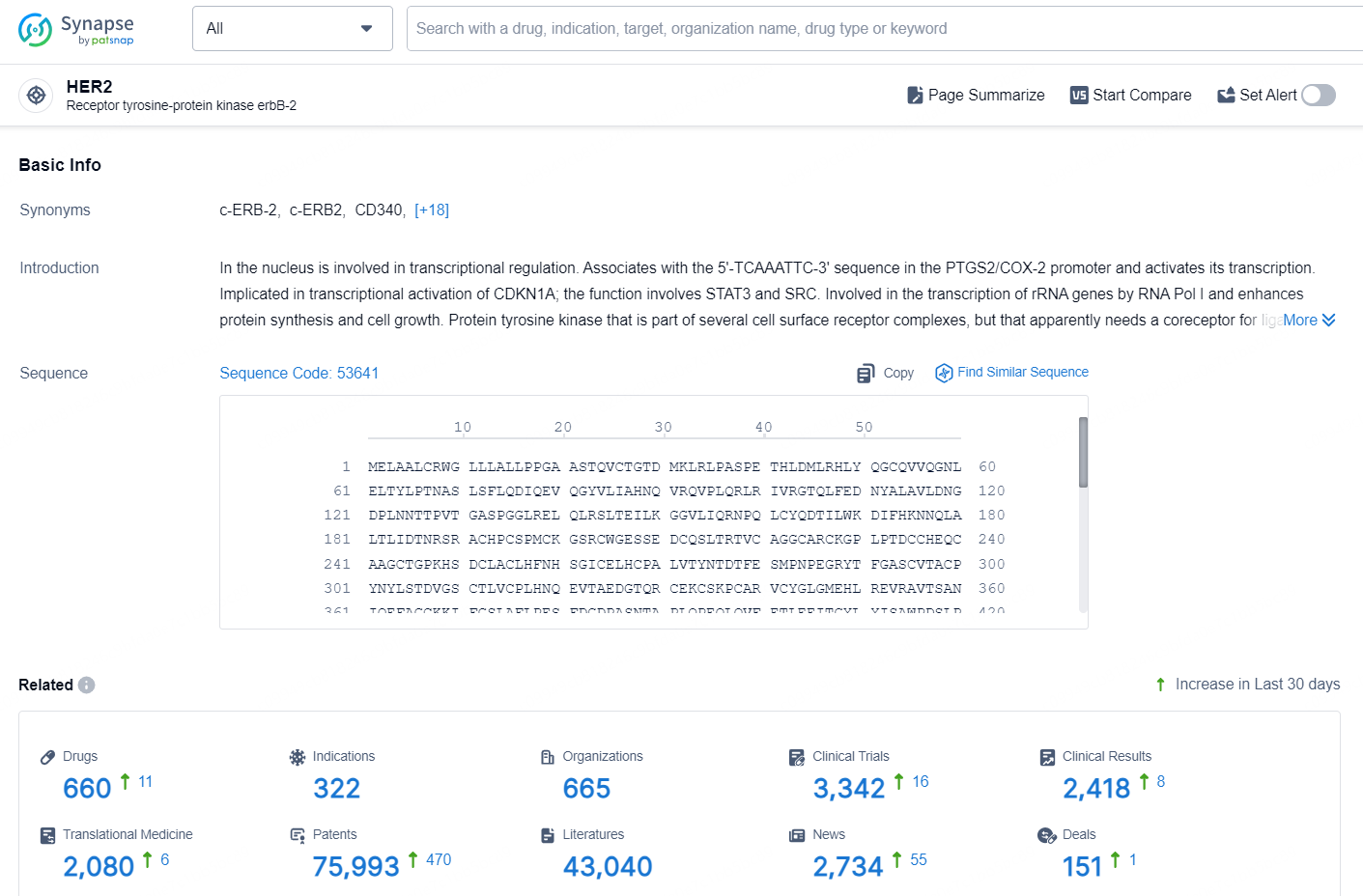
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 12, 2024, there are 660 investigational drugs for the HER2 targets, including 322 indications, 665 R&D institutions involved, with related clinical trials reaching 3342, and as many as 75993 patents.
Zongertinib is a small molecule drug that targets the HER2 receptor and has shown potential in the treatment of various types of cancer and respiratory diseases. The drug is being developed by Boehringer Ingelheim International GmbH and has reached the highest phase of clinical development, which is Phase 3, both globally and in China.