Initial Recipient of Omisirge™ (omidubicel-onlv) by Gamida Cell Administered to First Patient
Gamida Cell Ltd., a trailblazer in cell therapy aiming to transform cells into potent treatments, has declared that the initial patient has been subjected to a stem cell transplantation procedure using Omisirge (omidubicel-onlv).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Reaching this stage is a pivotal point for Gamida Cell and it propels us forward in our objective to offer potentially life-changing therapies to cancer patients," said Abbey Jenkins, Gamida Cell's CEO and President. “The introduction of Omisirge as a new source for stem cell transplant donors instills fresh optimism in many patients hoping for a cure. Our work centers around bringing this difference in the lives of cancer sufferers.”
The FDA granted Omisirge approval in April 2023 for use with adult and pediatric patients aged 12 and over with hematologic malignancies, with the intent of an umbilical cord blood transplant after myeloablative conditioning, aiming to decrease the neutrophil recovery period and the risk of infection.
Exceeding its target for the 2023 campaign, Gamida Cell has successfully brought on board 15 transplant centers throughout the U.S., securing coverage from healthcare insurers that cater to 90% of the commercial market. Actively partnering with over 90% of the top 70 transplant centers, known to carry out roughly 80% of transplants, Gamida Cell shows an upward trend in the enrollment in its Gamida Cell Assist® signaling program, indicating the intention of transplanters to use Omisirge as the donor resource.
"Omisirge's market launch has shown promise with regards to payer coverage, adopting transplant centers, and the interest shown by transplanters in using Omisirge as a donor source," said Michele Korfin, Gamida Cell's Chief Commercial Officer and COO. "Feedback from transplanters suggesting that Omisirge could increase donor source accessibility to more patients and improve upon existing donor source limitations is encouraging.”
Each year, about 8,000 stem cell transplants are carried out on patients with hematologic malignancies in the U.S., with an additional 1,700 patients deemed transplant-eligible, yet unable to locate a suitable donor. Historically, locating a matching donor is more difficult for ethnically diverse populations compared to white patients. Market research by Gamida Cell hints that Omisirge could potentially capture approx 20% of the allogeneic stem cell transplant market share by the year 2028.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
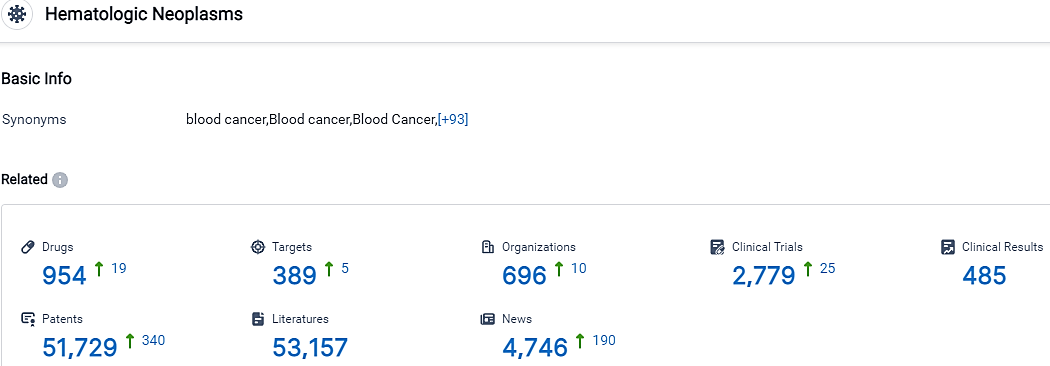
According to the data provided by the Synapse Database, As of October 5, 2023, there are 954 investigational drugs for the Hematologic Neoplasms, including 389 targets, 696 R&D institutions involved, with related clinical trials reaching 2779,and as many as 51729 patents.
Omisirge has been sanctioned to treat a range of blood disorders such as anemia, aplastic, myelodysplastic syndromes, hematologic neoplasms, hemoglobinopathies, sickle cell anemia, and thalassemia. It has reached the top level of its development phase, and its first official approval came from the United States in April 2023. Not only that, Omisirge has also been granted priority review, orphan drug, and breakthrough therapy statuses, showcasing its prospective medicinal value and importance.