Regadenoson: Detailed Review of its Transformative R&D Success
Regadenoson's R&D Progress
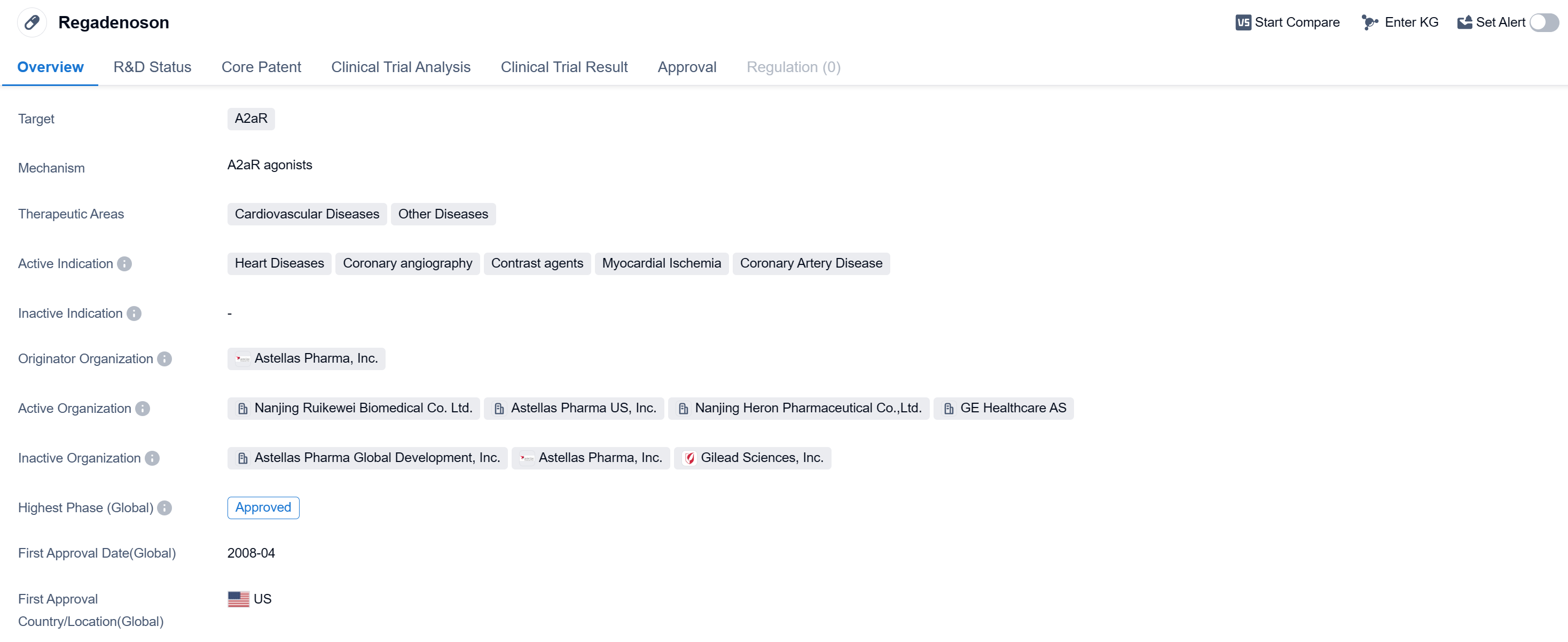
Regadenosonis a small molecule drug that targets the A2aR receptor. It is primarily used in the treatment of cardiovascular diseases, including heart diseases, coronary angiography, contrast agents, myocardial ischemia, and coronary artery disease. The drug was developed by Astellas Pharma, Inc., a pharmaceutical company.
Regadenoson has received approval for use in the global market. Its highest phase of development, which is the phase at which it received approval, is approved. The drug was first approved in the United States in April 2008.
As a small molecule drug, regadenoson is designed to interact with the A2aR receptor, which is involved in various physiological processes related to cardiovascular health. By targeting this receptor, regadenoson aims to modulate its activity and provide therapeutic benefits in the treatment of cardiovascular diseases.
The therapeutic areas in which regadenoson is indicated include cardiovascular diseases and other diseases. This suggests that the drug may have potential applications beyond just heart-related conditions, although the specific details of these other diseases are not provided.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Regadenoson: A2aR agonists
A2aR agonists refer to substances or drugs that activate the A2a adenosine receptor (A2aR). The A2a adenosine receptor is a protein found on the surface of cells, particularly in the central nervous system and immune cells. When activated by specific molecules, such as A2aR agonists, the receptor triggers a series of cellular responses.
From a biomedical perspective, A2aR agonists have been studied for their potential therapeutic applications. Activation of the A2a adenosine receptor has been shown to have anti-inflammatory and immunosuppressive effects. Therefore, A2aR agonists are being investigated as potential treatments for various conditions, including autoimmune diseases, neurodegenerative disorders, and cancer.
By binding to the A2a adenosine receptor, these agonists can modulate immune responses and reduce inflammation. This can help in controlling excessive immune reactions and mitigating the damage caused by chronic inflammation. Additionally, A2aR agonists have shown promise in enhancing the efficacy of cancer immunotherapy by promoting anti-tumor immune responses.
It is important to note that the specific mechanism of action and potential therapeutic benefits of A2aR agonists may vary depending on the specific drug or compound being used. Ongoing research aims to further explore the potential of A2aR agonists in various biomedical applications.
Drug Target R&D Trends for Regadenoson
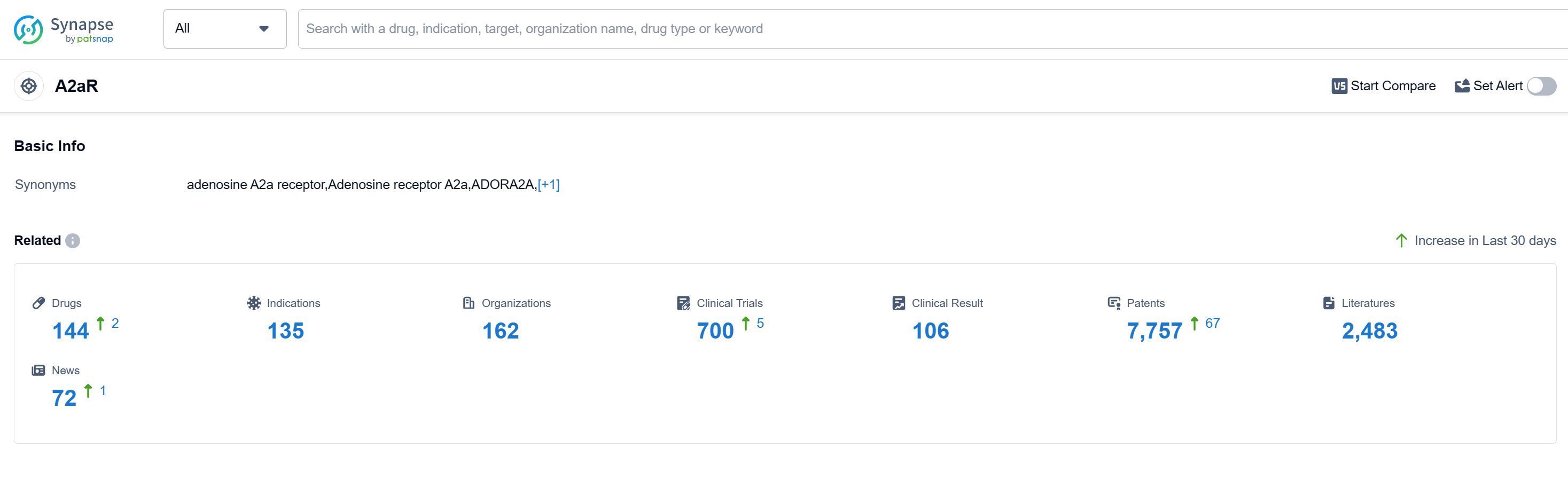
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 144 A2aR drugs worldwide, from 162 organizations, covering 135 indications, and conducting 700 clinical trials. The analysis of the current competitive landscape of target A2aR reveals that several companies are growing rapidly and have drugs in various stages of development. The highest stage of development is the "Approved" phase, indicating successful completion of clinical trials and approval for use. The indications for the approved drugs cover a wide range of conditions, including Common Cold, Headache, Pain, Migraine Disorders, Neuralgia, Fever, Dysmenorrhea, Asthma, Cardiovascular Diseases, Toothache, Parkinson Disease, Tension-Type Headache, Low Back Pain, Bronchitis, Bronchiectasis, Pain, Postoperative, Nasal Obstruction, Perioperative ischaemia, Influenza, Human, and Cough. The most rapidly progressing drug type under this target is Small molecule drugs, with biosimilars in the form of Monoclonal antibodies also ranking high. China is the country with the highest number of approved drugs under this target, indicating significant progress in drug development. Overall, the future development of target A2aR looks promising with a diverse range of companies, indications, drug types, and countries/locations involved in its research and development.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, regadenoson is a small molecule drug developed by Astellas Pharma, Inc. that targets the A2aR receptor. It has been approved for use in the treatment of cardiovascular diseases and other diseases. Its approval in the global market highlights its potential as a therapeutic option for patients suffering from heart-related conditions.