InnoCare reports positive Phase II results for TYK2 inhibitor ICP-488 in psoriasis patients
InnoCare Pharma (HKEX: 09969; SSE: 688428), a prominent biopharmaceutical firm specializing in cancer and autoimmune disease therapies, disclosed that the phase II clinical trial findings for the innovative TYK2 (Tyrosine Kinase 2) inhibitor ICP-488 achieved the main objective in adult individuals suffering from moderate-to-severe plaque psoriasis.
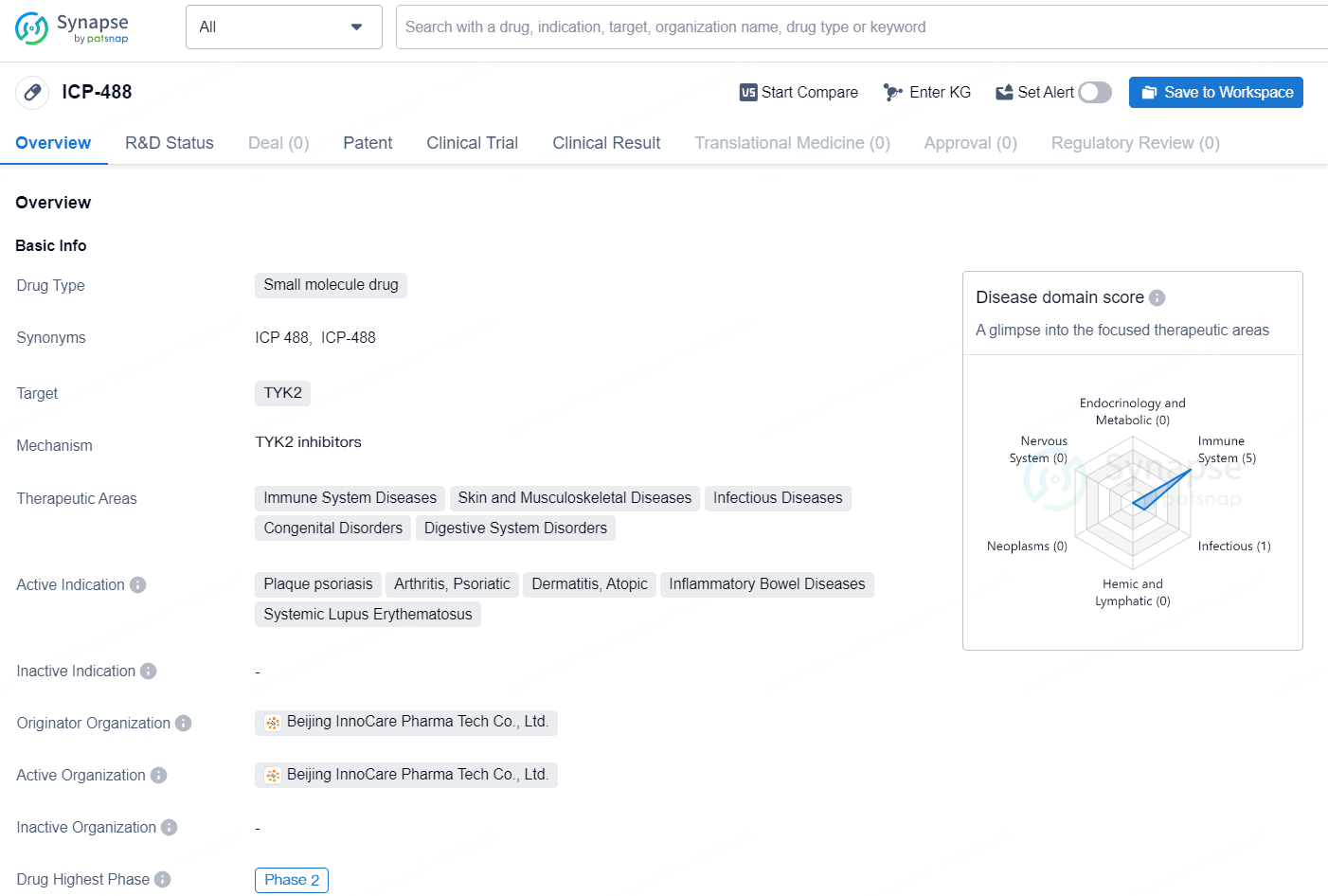
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In a clinical study involving psoriasis patients treated with ICP-488 over a 12-week period, the drug exhibited a strong efficacy and safety profile. ICP-488 successfully met various efficacy objectives, including Psoriasis Area and Severity Index (PASI) 75, PASI 90, PASI 100—indicating at least 75%, 90%, and 100% improvements in PASI scores from the baseline—as well as static Physician’s Global Assessment (sPGA) 0/1, demonstrating scores of 0 ('clear') or 1 ('almost clear') for both the 6mg and 8mg dose groups.
Specifically, after 12 weeks of treatment with once-daily doses of either 6mg or 9mg of ICP-488, PASI 75 was achieved by 77.3% and 78.6% of participants, respectively, compared to just 11.6% in the placebo group (p<0.0001). Moreover, PASI 90 was seen in 36.4% and 50.0% of patients, while none in the placebo cohort (p<0.0001); similarly, PASI 100 was recorded in 11.4% and 11.9% respectively, with 0% in the placebo group (p<0.05). The sPGA 0/1 rate was recorded at 70.5% and 71.4%, in contrast to 9.3% for the placebo group (p<0.0001).
ICP-488 showed good tolerability and an overall positive safety profile, with most treatment-emergent adverse events (TEAEs) and treatment-related adverse events (TRAEs) categorized as mild to moderate.
Dr. Jasmine Cui, Co-founder, Chairwoman, and CEO of InnoCare, expressed, “Psoriasis necessitates ongoing management, and there is a considerable unmet clinical requirement for innovative therapies. We are thrilled to witness the favorable outcomes from the phase II trial of ICP-488, and we will expedite its clinical progression to assist patients with psoriasis and other autoimmune conditions.”
This study was a multicenter, randomized, double-blind, placebo-controlled phase II clinical trial aimed at assessing the efficacy, safety, pharmacokinetic (PK), and pharmacodynamic (PD) properties of ICP-488 in Chinese adults diagnosed with moderate to severe plaque psoriasis. A total of 129 participants were included, allocated randomly into three treatment arms in a 1:1:1 ratio: a 6mg once-daily group, a 9mg once-daily group, and a placebo group, all undergoing treatment for 12 continuous weeks.
ICP-488 functions as an oral, potent, and selective allosteric inhibitor of TYK2. It binds to the JH2 domain and obstructs the signal transduction pathways of IL-23, IL-12, type 1 IFN, and other inflammatory cytokines, thereby impeding the harmful progression associated with autoimmune and inflammatory diseases.
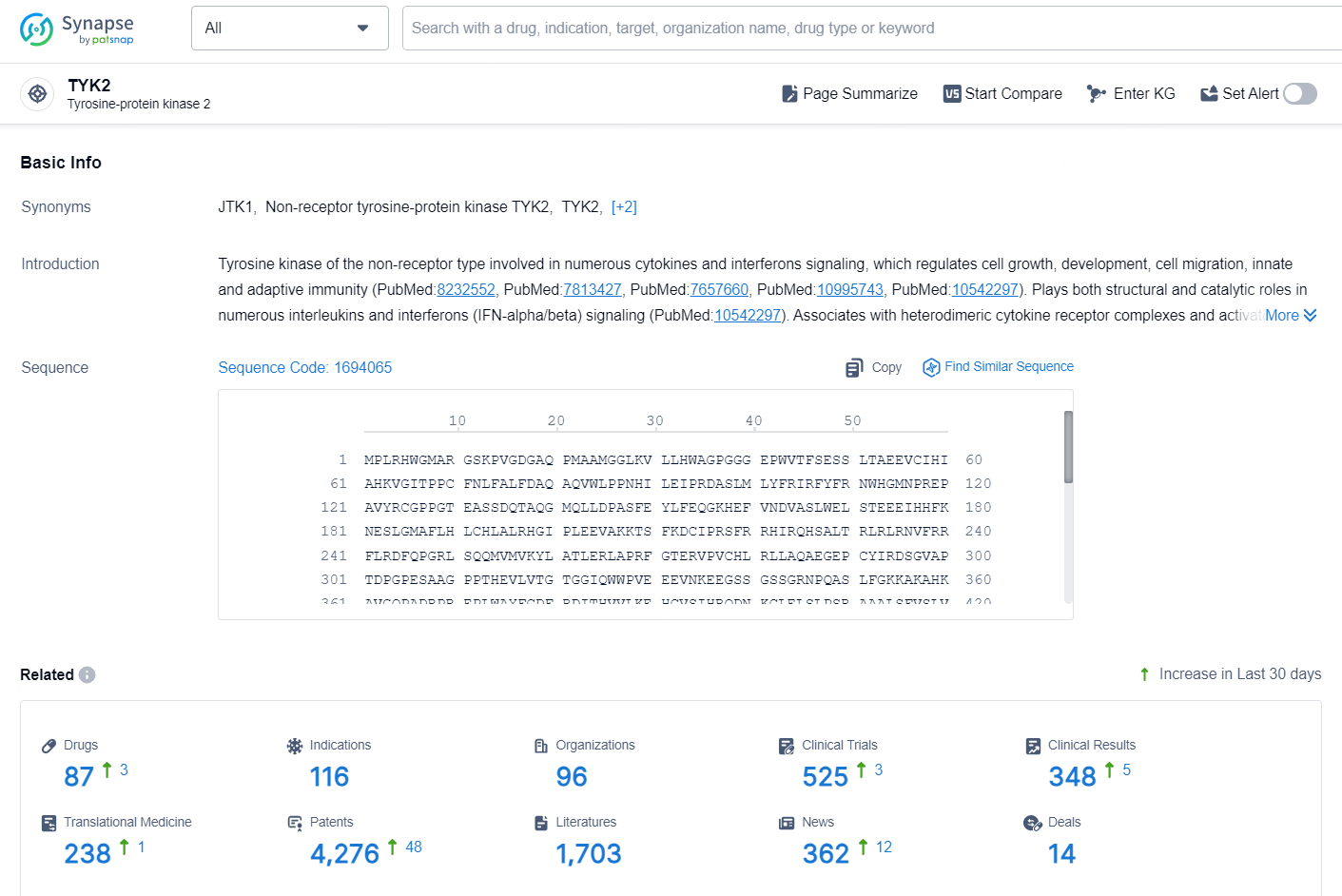
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 10, 2024, there are 87 investigational drugs for the TYK2 target, including 116 indications, 96 R&D institutions involved, with related clinical trials reaching 525, and as many as 4276 patents.
The small molecule drug ICP-488 is developed by Beijing InnoCare Pharma Tech Co., Ltd. and its main target is TYK2. This drug is primarily focused on treating a wide range of diseases related to the immune system, skin and musculoskeletal disorders, infectious diseases, congenital disorders, and digestive system disorders. The active indications for ICP-488 include plaque psoriasis, arthritis (psoriatic), dermatitis (atopic), inflammatory bowel diseases, and systemic lupus erythematosus.