Teva Submits Biosimilar of Prolia® (Denosumab) to U.S. FDA and EU EMA for Review
Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) has revealed that the U.S. Food and Drug Administration (FDA) has accepted and the European Medicines Agency (EMA) has confirmed the applications for TVB-009P, a biosimilar candidate for Prolia® (denosumab). The submissions include a Biologics License Application (BLA) in the U.S. focusing on interchangeability, as well as a Marketing Authorization Application (MAA) in the European Union (EU). Both applications cover all indications that have been approved for the reference product, Prolia, addressing conditions that have a significant fracture risk, such as osteoporosis in postmenopausal women. The FDA is expected to make a decision and the EMA is anticipated to provide its opinion in the latter half of 2025.
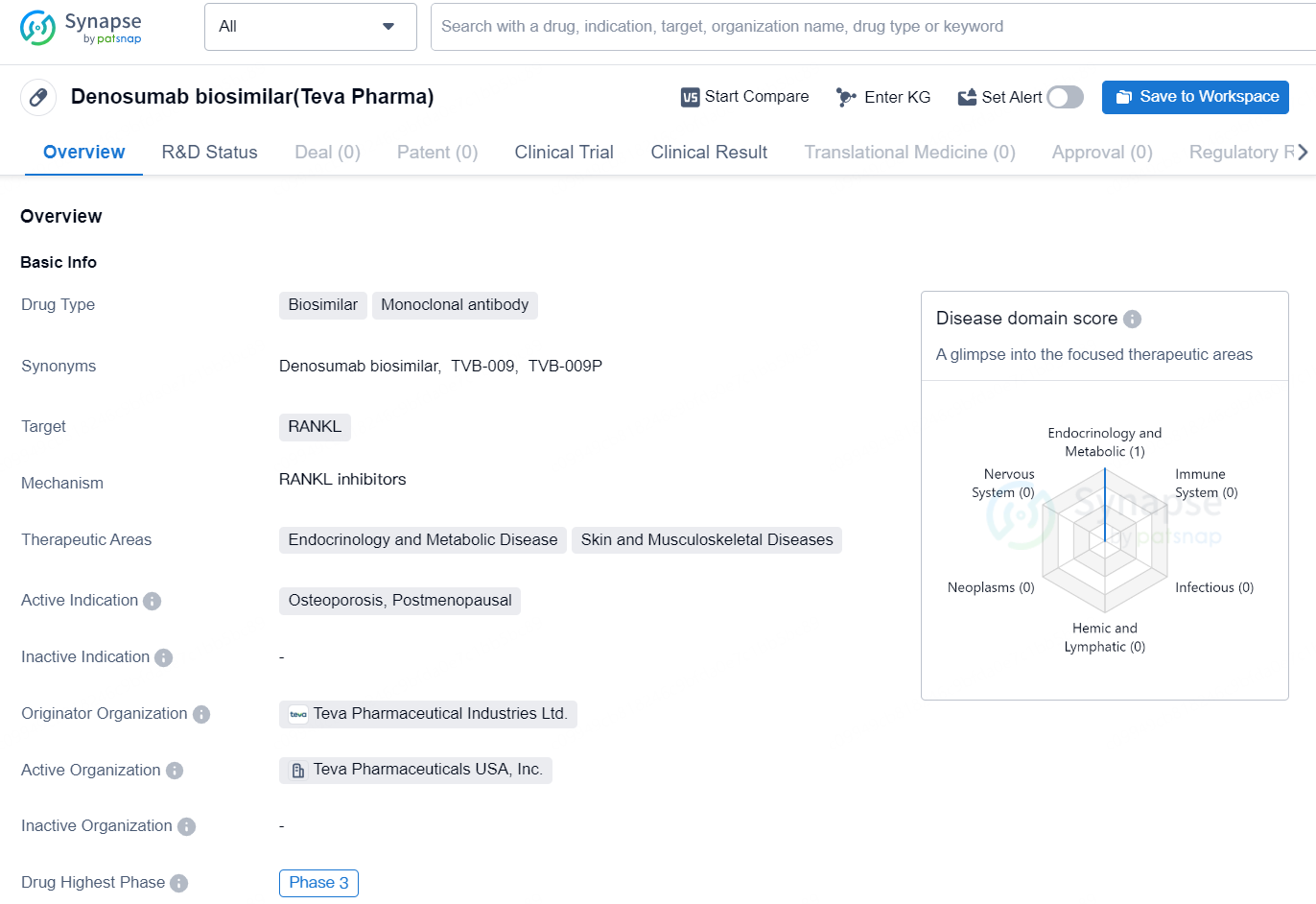
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
TVB-009P, Teva's proposed biosimilar for Prolia, marks the company's initial submission of an internally developed biosimilar to the U.S. FDA. This submission is underpinned by an extensive clinical and analytical data compilation, which includes findings from the randomized, double-blind Phase 3 trial, TVB009-IMB-30085, assessing the safety and effectiveness of TVB-009P in comparison to Prolia for females experiencing postmenopausal osteoporosis. Additional data from the pharmacokinetics and pharmacodynamics study, TVB009-BE-10157, conducted in healthy subjects, indicated pharmacokinetic similarity with the reference product.
With a legacy spanning more than 120 years in delivering accessible and cost-effective medications, Teva stands as a prominent player in the biosimilar sector, possessing over 20 biosimilars within its portfolio and pipeline. “The acceptance and validation of our filing for TVB-009P, the proposed biosimilar to Prolia, reflects Teva's dedication to expanding global biosimilar access in various markets,” stated Steffen Nock, PhD, who serves as Head of Biosimilars and Chief Scientific Officer at Teva. “We aim to utilize our extensive expertise in generics alongside our proven success in biologics, exemplified by AJOVY®, to foster growth within the biosimilars space. Our objective is to broaden our strategic collaborations and enrich our portfolio, thus providing patients with more affordable therapeutic alternatives.”
In the U.S. and Europe, over 165 million women are either menopausal or postmenopausal. The hormonal shifts that occur during menopause significantly elevate the risk of developing osteoporosis, which affects approximately 25% of older women in these regions. Osteoporosis heightens the likelihood of bone fractures, with an estimated one in three women aged 50 and above expected to experience a fracture as a result of this condition.
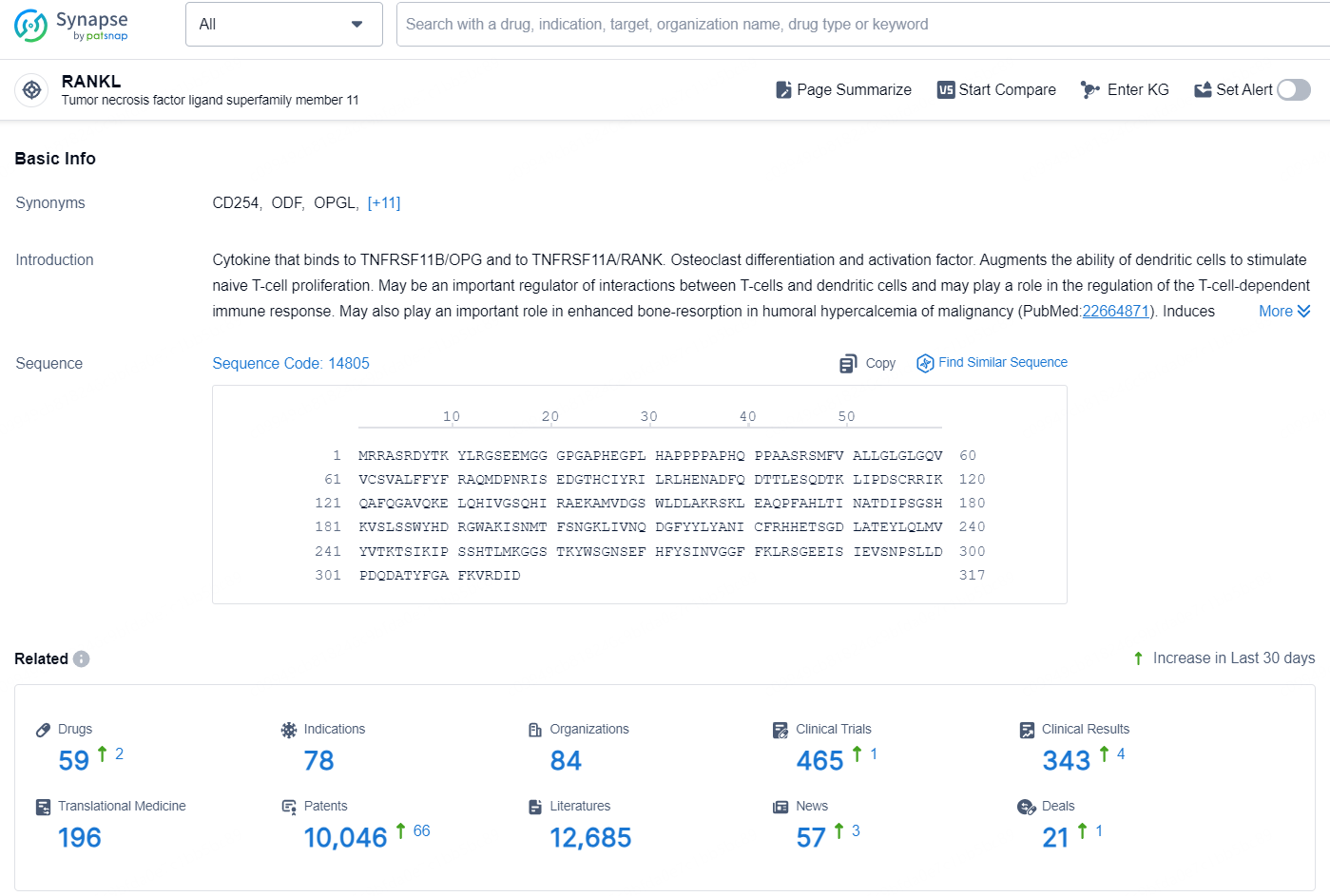
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 11, 2024, there are 59 investigational drugs for the RANKL targets, including 78 indications, 84 R&D institutions involved, with related clinical trials reaching 465, and as many as 10046 patents.
The Denosumab biosimilar developed by Teva Pharmaceutical Industries Ltd. is a monoclonal antibody and falls under the category of biosimilar drugs. This drug targets RANKL and is designed to treat conditions within the therapeutic areas of endocrinology and metabolic disease, as well as skin and musculoskeletal diseases. The active indication for this medication is the treatment of osteoporosis in postmenopausal women.