InnoCare Reveals Positive Outcome for Phase 2 Trial of TYK2 Blocker ICP-332 in Atomic Dermatitis Subjects, Achieving Main Goal
InnoCare Pharma, an innovative biotechnology firm specialized in creating therapies for oncology and immunological disorders, has publicized that the phase II clinical trial outcomes for the groundbreaking TYK2 inhibitor ICP-332 have successfully achieved the main goal in treating adult individuals who suffer from moderate to severe forms of atopic dermatitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
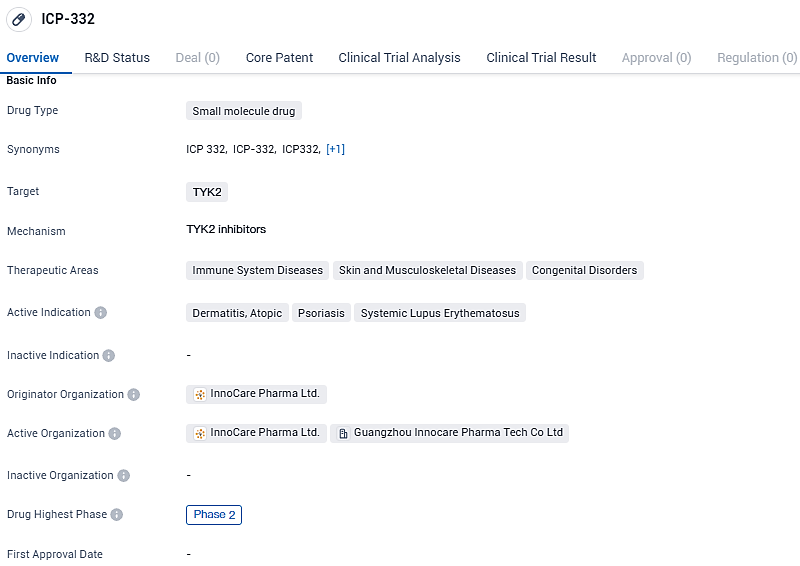
After a 4-week treatment phase, patient studies indicated that ICP-332 has shown notable effectiveness and a favorable safety profile in managing symptoms of Alzheimer's Disease (AD). The drug met various metrics of success, such as accomplishing the Eczema Area and Severity Index (EASI) scores of 50, 75, and 90, as well as receiving a score of 0/1 on the Investigator’s Global Assessment, with these outcomes observed in both the 80mg and 120mg dosage groups.
In terms of improvements measured by the EASI score, the average reduction from the initial scores was an impressive 78.2% for the 80mg group and 72.5% for those taking 120mg daily, each group displaying a statistically significant advantage over the placebo, which showed a change of only 16.7%. Furthermore, 64% of patients in both the 80mg and 120mg cohorts achieved an EASI 75 rating, far outpacing the 8% who achieved this marker on the placebo, with these differences holding significant weight (p<0.0001).
The safety and tolerability of ICP-332 also compared well against the placebo, with any adverse events linked to treatment being classified as either mild or moderate. Dr. Jasmine Cui, who holds multiple leadership positions at InnnoCare including Co-founder, Chairwoman, and CEO, commented on the matter with optimism: "AD represents a significant gap in current medical treatment options, and the promising outcomes of this phase II ICP-332 study have us hopeful. We're aiming to expedite the advancement of this clinical research in hopes of aiding those suffering from AD, among other autoimmune conditions."
Currently, the global market lacks an approved TYK2 inhibitor specifically for AD. Insights from Pharma Intelligence forecast that AD is on the rise as a prominent autoimmune disorder, with market potential projected to reach around $10 billion by the year 2030.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
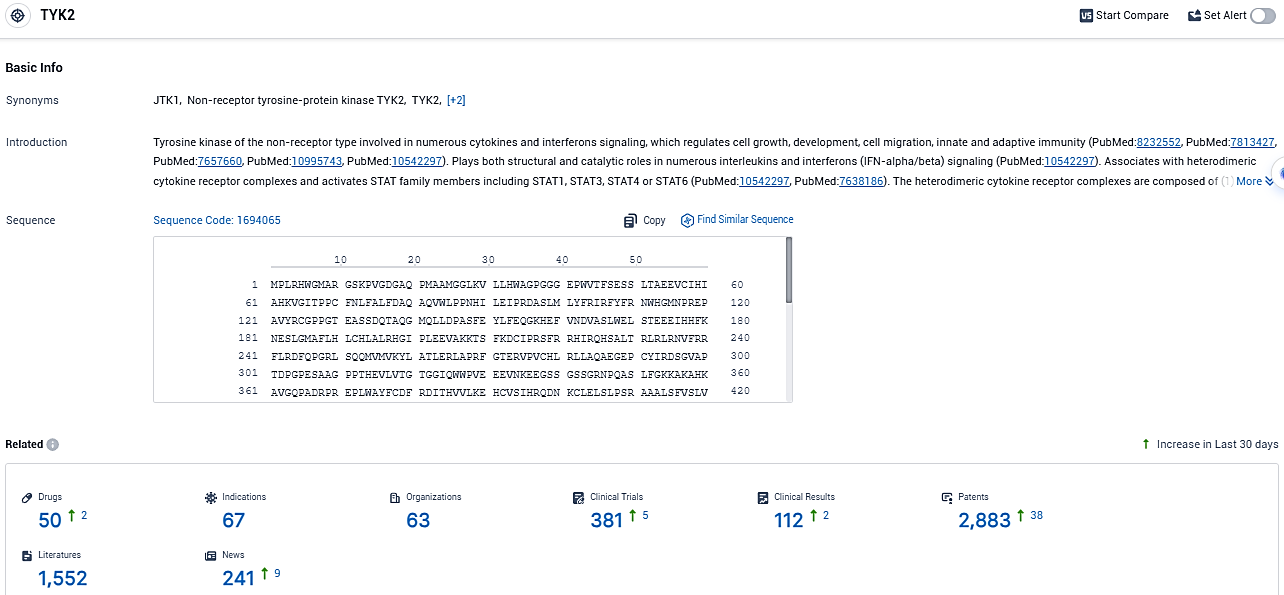
According to the data provided by the Synapse Database, As of December 27, 2023, there are 50 investigational drugs for the TYK2 target, including 67 indications, 63 R&D institutions involved, with related clinical trials reaching 381, and as many as 2883 patents.
ICP-332 is a small molecule drug that targets TYK2 and is being developed for the treatment of immune system diseases, skin and musculoskeletal diseases, and congenital disorders. It has shown promising results in Phase 2 trials and is being further evaluated for its safety and efficacy.