Internationalization Wave of Chinese Antibody Drugs in January 2025
In January 2025, a series of collaborative agreements were successively reached in the field of antibody-based pharmaceuticals in China: WuXi Biologics partnered with Candid Therapeutics to advance the development of a BCMA, CD20, and CD19 tri-specific antibody therapy through its WuXiBody™ platform. This groundbreaking partnership indicates a new direction for future oncological immunotherapy. Concurrently, Keymed Biomedical's CD38 monoclonal antibody, CM313, was successfully licensed to Timberlyne Therapeutics, further solidifying China's leading position in the treatment of hematological malignancies.
These agreements not only demonstrate China's profound expertise in antibody engineering and drug development but also reflect the determination of Chinese enterprises to expand into international markets and seek global development. For instance, Kelun Botai's collaboration with Harbour BioMed on the TSLP monoclonal antibody SKB378/HBM9378 received investment and cooperation from Windward Bio AG, aimed at addressing unmet needs in respiratory diseases such as asthma and chronic obstructive pulmonary disease. Moreover, the collaboration between Simcere Zaiming and AbbVie brings new hope to patients with multiple myeloma. SIM0500, a GPRC5D/BCMA/CD3 tri-specific antibody, with its unique targeting mechanism, is poised to be an innovator in the field.
In this wave of international expansion, the international licensing of the bispecific antibody ICP-B02 (CM315) is also noteworthy. InnoCare Pharma and Keymed Biomedical joined hands with Prolium Bioscience to promote the global commercialization of this CD20×CD3 bispecific antibody. This not only challenges traditional cancer treatment methods but also tests China's innovative capabilities and scientific research levels. Through such transnational cooperation, China's biopharmaceutical industry is gradually advancing to the forefront of the world, bringing more treatment options to patients in China and globally.
1.WuXi Biologics and Candid Therapeutics join forces to advance innovative tri-specific antibody therapy
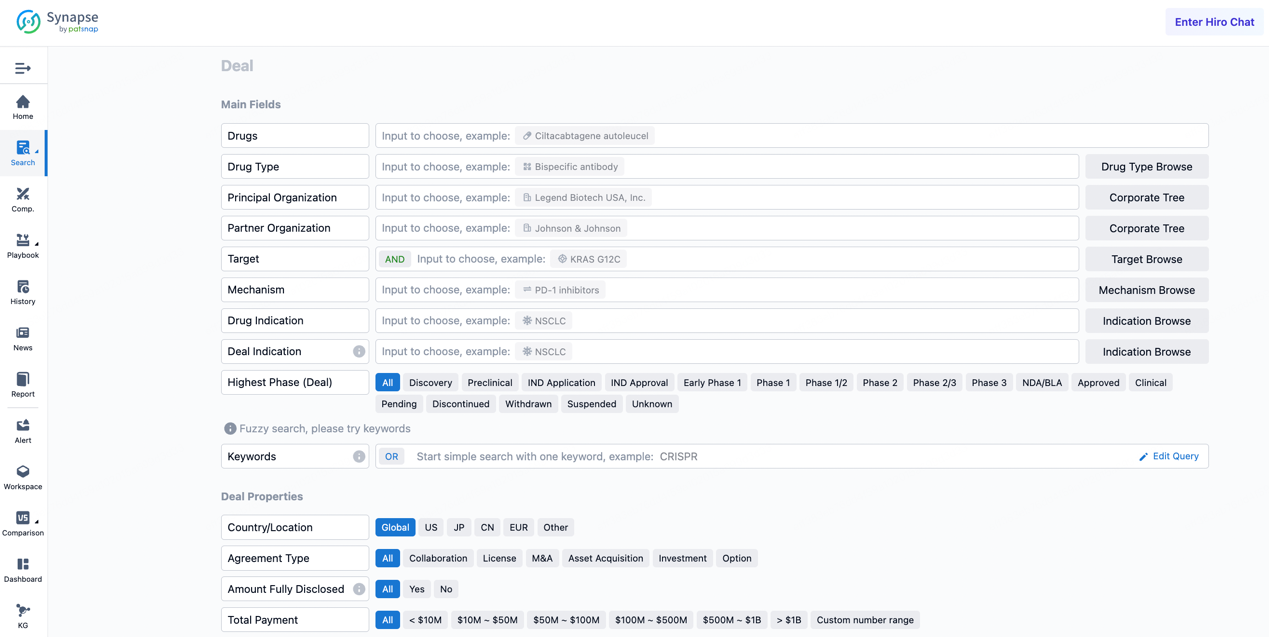
On January 7, 2025, WuXi Biologics and Candid Therapeutics entered into a significant research service collaboration agreement, marking a new phase in the development of innovative therapies. Under the terms of the agreement, Candid will acquire global rights to a tri-specific antibody developed on WuXi Biologics’ WuXiBody™ platform. This antibody, in the preclinical development stage, targets three different antigen sites: BCMA, CD20, and CD19. These targets are carefully chosen to enhance the killing effect on tumor cells via T-cell-mediated immune responses. BCMA (B-cell maturation antigen), highly expressed in multiple myeloma (MM) and other hematologic malignancies, is a key target for treating these diseases. As a member of the tumor necrosis factor receptor superfamily, BCMA is primarily expressed on the surface of malignant and normal plasma cells and is not expressed in essential tissues, making it an ideal treatment target. CAR-T cell therapies targeting BCMA have shown significant efficacy in relapsed or refractory MM patients, with overall response rates from 73% to 100% and complete response rates from 31% to 69%.
BCMA signaling pathway1
On the other hand, CD20 and CD19 are antigens broadly and relatively stably expressed on the surface of B cells, playing crucial roles in various B-cell-related cancers like non-Hodgkin's lymphoma (NHL). CD19 is an antigen that remains expressed throughout the B-cell development process and is also highly conserved in most B-cell malignancies. At the same time, CD20 is a marker of mature B cells, used as a target in the treatment of B-cell lymphomas and leukemias.
The uniqueness of WuXi Biologics’ tri-specific antibody lies in its ability to bind simultaneously to two different antigens on tumor cells (like CD20 and CD19) and an activation molecule on the surface of T cells (such as BCMA or possibly another co-stimulatory molecule). This multi-binding approach helps to direct T cells close to tumor cells and promote close contact between them, thereby enhancing T-cell-mediated cytotoxicity. Additionally, this tri-targeting could inhibit tumor cells' ability to evade immune surveillance, further increasing the effectiveness of the treatment. Notably, the WuXiBody™ platform's unique advantage is its ability to construct multi-specific antibodies of various valences to meet the biological needs of different projects, thus providing greater flexibility and potential for drug development.
The financial terms involved in this collaboration are also notable. WuXi Biologics will receive an undisclosed upfront payment and is eligible for subsequent development and sales milestone payments totaling up to $925 million (approximately RMB 6.78 billion), plus sales royalties post-commercialization. Such a transaction structure not only reflects the partners' confidence in the future market prospects of this tri-specific antibody but also provides WuXi Biologics with substantial economic returns.
2.Keymed Biomedical's CD38 Monoclonal Antibody CM313
On January 10, 2025, Keymed Biomedical (02162.HK) announced a significant agreement involving the exclusive licensing of global rights (excluding mainland China, Hong Kong, Macau, and Taiwan) for their autonomously developed humanized monoclonal antibody CM313 targeting CD38, to Timberlyne Therapeutics. CM313 is an innovative therapeutic agent that targets the CD38 molecule, a glycoprotein highly expressed on the surface of various hematologic tumor cells. As a potential "best-in-class" candidate, CM313 uniquely binds to the CD38 molecule to not only eradicate antibody-secreting cells, including long-lived plasma cells (LLPCs), thereby preventing antibody-mediated platelet destruction but also restoring normal platelet production and function. Furthermore, CM313 has the ability to suppress the activity of immune cells such as monocytes, demonstrating significant potential in treating conditions like relapsed/refractory multiple myeloma, lymphoma, and other autoimmune diseases.
CM313 is capable of directly inducing apoptosis in tumor cells and indirectly killing them through mechanisms such as Antibody-Dependent Cellular Cytotoxicity (ADCC), Complement-Dependent Cytotoxicity (CDC), and Antibody-Dependent Cellular Phagocytosis (ADCP). Preclinical studies have shown that CM313 exhibits a therapeutic response rate of up to 95% in adult patients with primary immune thrombocytopenia (ITP) and has displayed favorable safety profiles. These results indicate that CM313 could be an effective treatment option, particularly for patients who are unresponsive or intolerant to conventional therapies. Currently, CM313 is in various stages of clinical trials to evaluate its efficacy and safety across different indications.
From a business perspective, this licensing deal marks a significant step for Keymed Biomedical in the international market. Under the terms of the agreement, Keymed Biomedical will receive an upfront payment of $30 million, near-term payments, and become the largest shareholder in Timberlyne, holding 25.79% of its equity. Moreover, upon achieving certain sales and development milestones, Keymed Biomedical is eligible to earn up to an additional $337.5 million in milestone payments, as well as tiered royalties based on sales. Concurrently, Timberlyne has completed a Series A financing round totaling $180 million, supported by prominent investment institutions including Bain Capital Life Sciences and Venrock Healthcare Capital Partners, providing essential financial backing for further research and development of CM313, and affirming high market confidence in the drug's prospects.
3.Kelun Botai and Harbour BioMed join forces, propelling TSLP monoclonal antibody SKB378/HBM9378 towards the global market
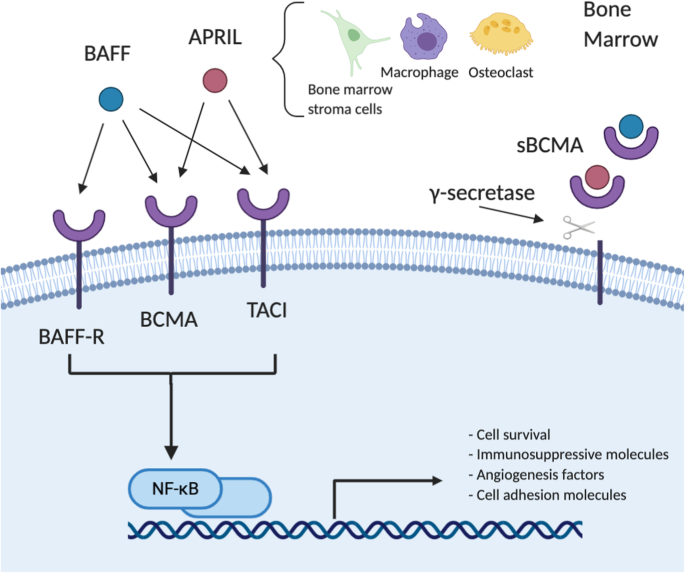
On January 10, 2025, Kelun Botai and Harbour BioMed jointly announced that they had entered into an important exclusive licensing agreement with Windward Bio AG for the jointly developed anti-thymic stromal lymphopoietin (TSLP) monoclonal antibody SKB378/HBM9378. This drug is a fully human monoclonal antibody targeting the critical TSLP, which functions by inhibiting the TSLP-mediated signaling pathways through blocking the interaction between TSLP and its receptor. TSLP is a cytokine playing a crucial role in the development and progression of various immunological diseases, including asthma and chronic obstructive pulmonary disease (COPD). When TSLP binds to its receptor, it initiates a series of complex downstream signaling cascades involving significant mechanisms such as the JAK-STAT pathway, thereby promoting inflammatory responses. Binding of TSLP to the complex of TSLP receptor (TSLPR) and IL-7Rα activates antigen-presenting cells and promotes the differentiation of naive CD4+ T lymphocytes into Th2 cells, leading to the release of pro-inflammatory cytokines like IL-4, IL-5, and IL-13. Excessive activation of this Th2-type immune response is a key driver of many allergic diseases, such as asthma, atopic dermatitis, and certain autoimmune diseases. In patients with allergic asthma, elevated TSLP levels produced by airway epithelial cells are significantly related to disease severity and decreased lung function. By inhibiting TSLP, SKB378/HBM9378 effectively blocks upstream inflammatory mediators, reduces the release of pro-inflammatory cytokines, alleviates airway inflammation and allergic reactions, and improves patient symptoms and lung function.
Functions of TSLP in promoting type 2 responses3
As a novel recombinant fully human monoclonal antibody, SKB378/HBM9378 not only exhibits high specificity and potent biological activity but has also been engineered to have an extended half-life, meaning that patients may require fewer dosing sessions to maintain therapeutic efficacy. Furthermore, in contrast to other drugs of the same type, it employs a subcutaneous injection method, enhancing the convenience of administration. SKB378/HBM9378 has already completed a Phase I clinical trial in China for moderate to severe asthma and has demonstrated good safety and preliminary efficacy. With the advancement of this collaboration, more clinical studies on COPD are planned, marking the drug as a potential new option for treating such severe respiratory diseases.
According to the terms of the agreement signed by the parties, Kelun Botai and Harbour BioMed will receive up to $970 million from Windward Bio in upfront and milestone payments, along with tiered royalties based on net sales ranging from single to double digits percentage. Specifically, the upfront and near term payments total $45 million, including cash consideration and equity in Windward Bio's parent company. This partnership will operate under the NewCo model, with Windward Bio assuming responsibility for the research, development, production, and commercialization of SKB378/HBM9378 globally (excluding Greater China as well as parts of Southeast and West Asia).
4.Simcere Zaiming collaborates with AbbVie to drive breakthroughs in multiple myeloma treatment
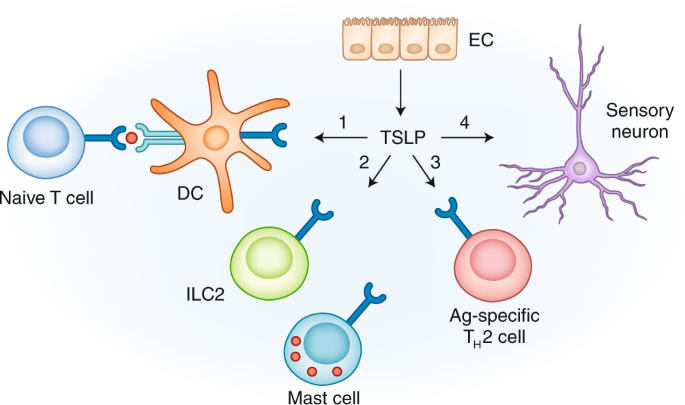
On January 13, 2025, Simcere Zaiming announced a significant development license option agreement with AbbVie. The collaboration centers around Simcere Zaiming's independently developed tri-specific antibody, SIM0500, which is currently in Phase I clinical studies in both China and the United States and has been granted Fast Track designation by the U.S. Food and Drug Administration (FDA). As a humanized tri-specific antibody targeting multiple myeloma (MM), SIM0500 uniquely targets G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D), B-cell maturation antigen (BCMA), and the CD3 molecule on T-cell surfaces. This multi-target approach enhances the selectivity and potency against tumor cells while improving treatment safety and efficacy.
Location and structure of GPRC5D4
SIM0500 features a low-affinity but highly target-specific CD3 antibody, which helps to avoid severe side effects such as cytokine release syndrome (CRS) often associated with traditional bispecific antibodies. Additionally, SIM0500 can recognize and bind to the highly expressed antigens GPRC5D and BCMA on malignant plasma cells, precisely directing the patient's immune system, especially T-cells, to attack the tumor cells. In preclinical studies, SIM0500 exhibited significant antitumor effects, good tolerability, lower effective doses, and sustained response after discontinuation, indicating its potential to be a leading candidate amongst treatments for multiple myeloma. These characteristics position SIM0500 to potentially overcome resistance issues with existing therapies, providing a novel and more effective treatment option for patients.
From a commercial perspective, this partnership represents a significant milestone for Simcere Zaiming. Under the terms of the agreement, Simcere Zaiming will receive an upfront payment from AbbVie and is eligible for up to USD 1.055 billion in option and milestone payments. Additionally, Simcere Zaiming will receive tiered royalties on net sales of the product outside of the Greater China area. This not only offers direct financial returns but also the opportunity to share in the global market success of SIM0500. AbbVie, in turn, will receive tiered royalties on net sales in the Greater China area. This collaborative model not only accelerates the development of SIM0500 but also reflects the high recognition and support of multinational pharmaceutical companies for innovative Chinese pharmaceutical enterprises.
5.Bispecific Antibody ICP-B02 (CM355): InnoCare Pharma Partners with Keymed Biomedical to Forge New Frontiers in Cancer Immunotherapy
On January 20, 2025, InnoCare Pharma and Keymed Biomedical jointly announced a significant collaboration involving a licensing agreement with Prolium Bioscience to develop and commercialize a bispecific antibody named ICP-B02 (CM355). ICP-B02 is an innovative therapeutic that can simultaneously recognize and bind to CD20 positive target cells and CD3 positive T cells. CD20 is an antigen predominantly found on the surface of B-cell lymphomas, while CD3 is a signature molecule on T cells, involved in the activation of these cells. This dual-targeting mechanism not only directs the immune system's T cells to the tumor cells but also activates them to enhance their ability to effectively kill cancer cells through a process known as T-cell-mediated cytotoxicity (TDCC).
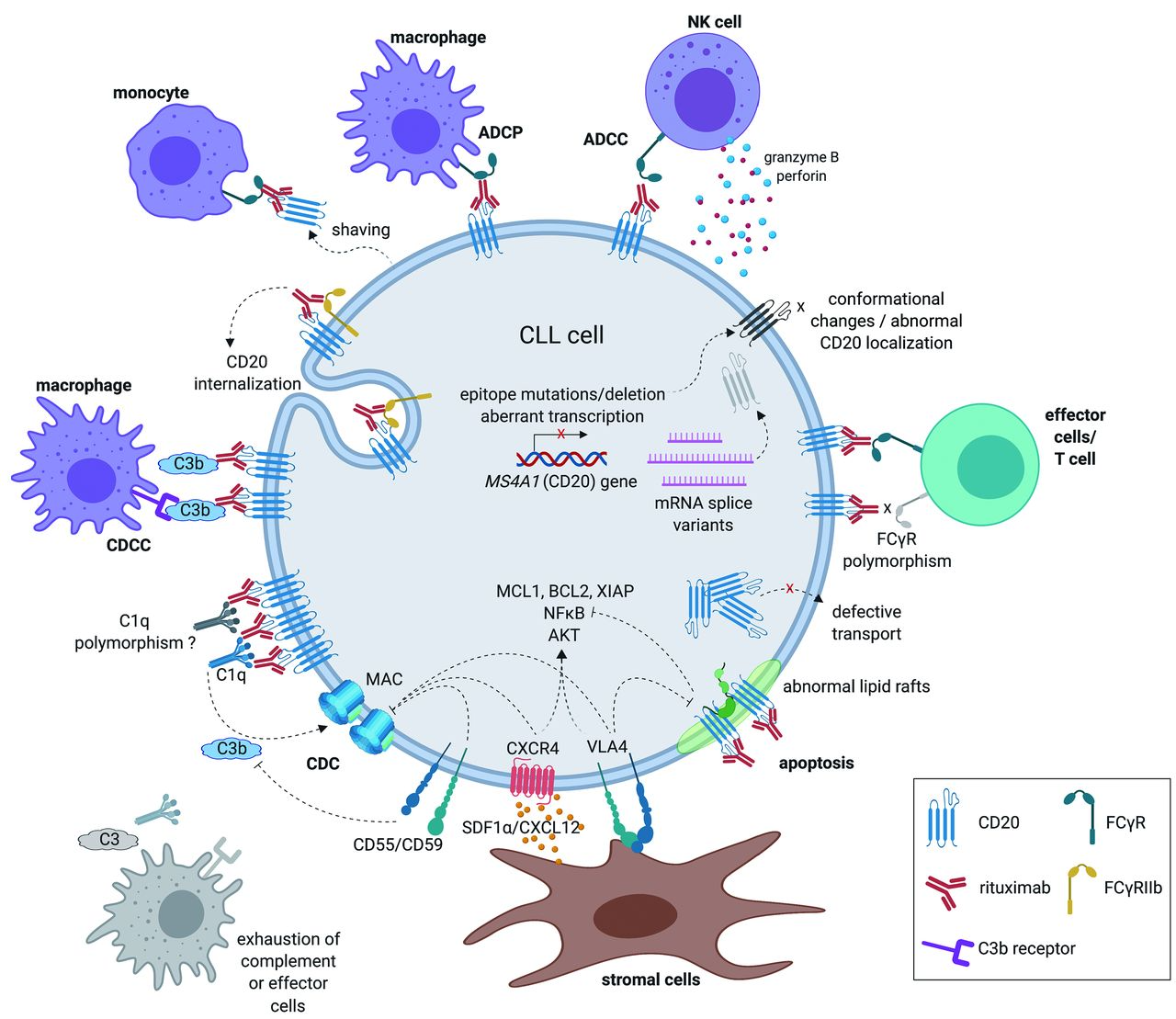
Summary of the known mechanisms of action of anti-CD20 monoclonal antibodies5
ICP-B02 exemplifies the modern trend in tumor immunotherapy – utilizing the body's own immune system to fight cancer through direct contact between cells. This physical proximity not only increases the immune system's ability to recognize tumor cells but also triggers a series of complex signal transduction events through crosslinking the T Cell Receptor (TCR) and CD3 complex. Initially, the CD3 molecules on the surface of T cells are activated under the multivalent cross-linking mediated by ICP-B02, leading to phosphorylation of intracellular motifs in the TCR/CD3 complex, thereby initiating downstream signaling pathways. These signals generally involve the activation of a series of kinases, including Lck and ZAP-70, which collectively enhance T-cell activity. Further, the activation of T cells induced by ICP-B02 is also accompanied by the activation of various effector functions, such as the release of cytokines and cytotoxic granules. In particular, T cells can secrete perforin and granzymes, which can penetrate the membranes of tumor cells and initiate programmed cell death. Moreover, ICP-B02 also promotes T-cell-mediated cytotoxicity (TDCC), where through direct cell-to-cell contact, T cells can effectively destroy neighboring tumor cells. Additionally, ICP-B02 has been optimized to reduce potential toxicity to normal tissues while enhancing therapeutic efficacy. Currently, ICP-B02 is undergoing Phase I/II clinical trials in China, primarily targeting patients with relapsed or refractory non-Hodgkin's lymphoma (NHL), including follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL). Preliminary results have demonstrated that ICP-B02, whether administered intravenously or subcutaneously, has shown good safety and significant antitumor activity in patients with these types of lymphoma.
This partnership brings significant economic value and development opportunities to InnoCare Pharma and Keymed Biomedical. According to the agreement, both companies will receive up to $520 million in total payments, including upfront payments, near-term payments, and additional payments upon reaching certain clinical development, registration, and commercialization milestones. Additionally, they will hold a minority stake in Prolium Bioscience and are entitled to receive tiered royalties on net sales of future products. Notably, Prolium has acquired the rights for development, registration, production, and commercialization in the global non-oncology domain and outside Asia in oncology. This deal not only reflects the international pharmaceutical industry's recognition of ICP-B02's potential but also signifies a solid step forward for InnoCare Pharma and Keymed Biomedical in expanding into the global market.
Summary
In review, examining the antibody drug collaborations in January 2025, we can see that Chinese pharmaceutical enterprises are focusing their research on highly expressed and critically important targets for both hematologic malignancies and respiratory diseases such as BCMA, CD20, CD19, and TSLP. These targets are not only significant for diagnosing diseases but are also crucial for developing precision treatment strategies. Therefore, the antibody design and clinical trials centered around these targets often achieve notable therapeutic effects, bringing new hope for cure to patients.
However, while pursuing innovation, balancing efficacy and safety remains a significant challenge for all researchers. For instance, although SIM0500 was designed to minimize the risk of side effects, continuous monitoring and evaluation are still required in its application. Considering the complexity and diversity of different indications, each novel antibody requires rigorous clinical validation to ensure its safety and efficacy, which is crucial for improving the overall quality of the medication and its competitive position in the market.
How to get the latest progress on drug deals?
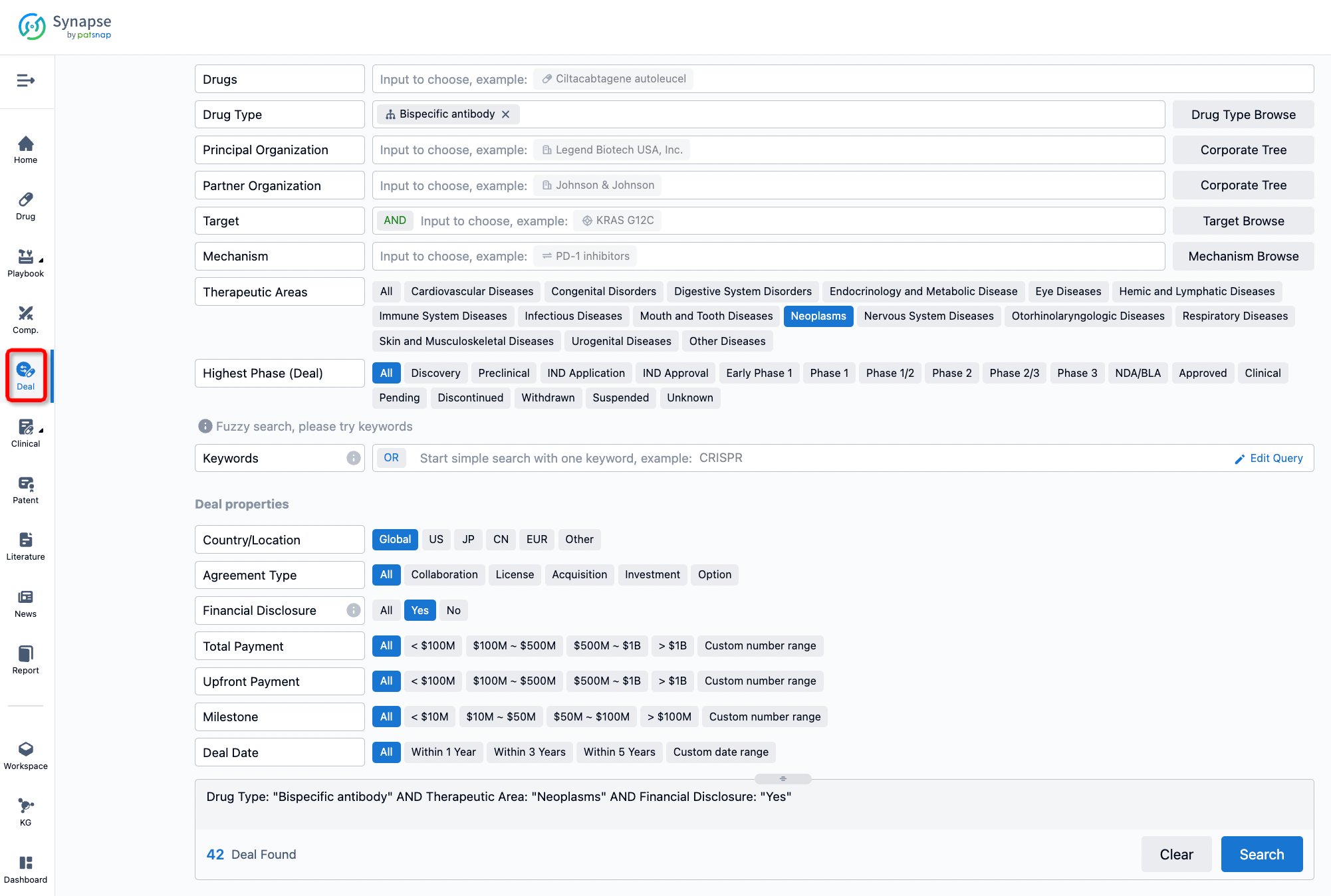
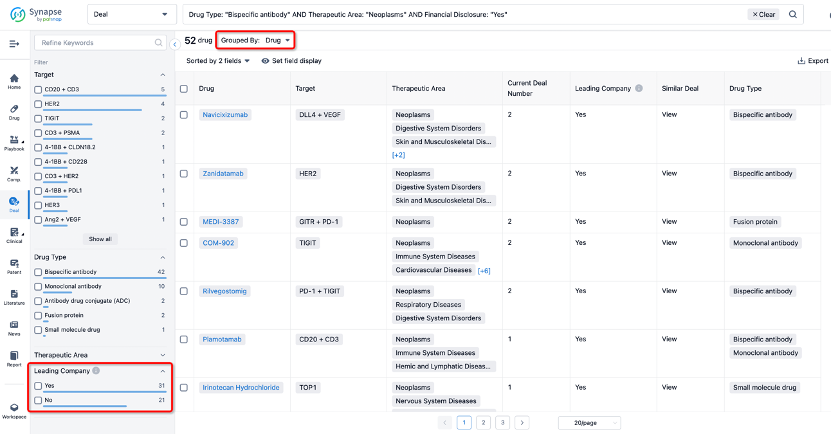
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
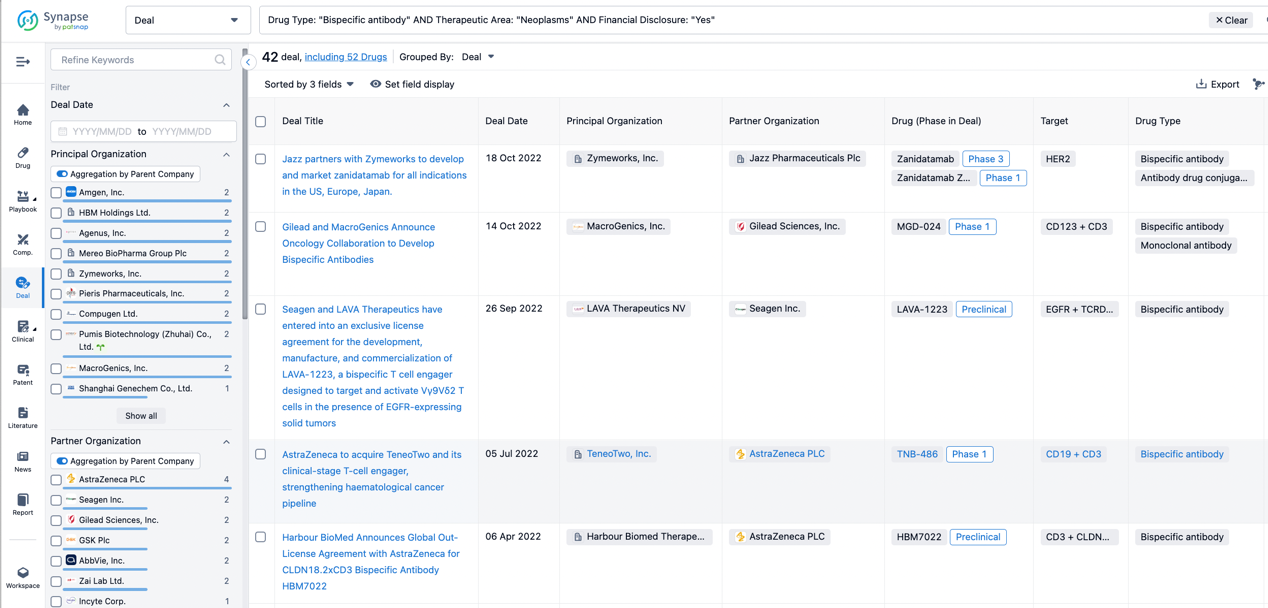
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
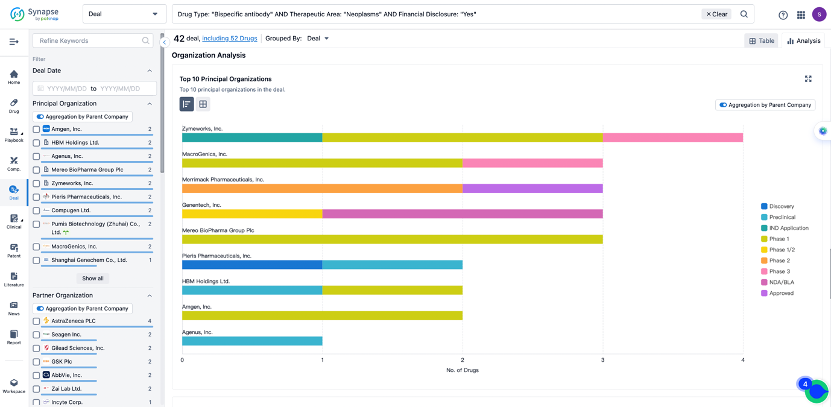
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
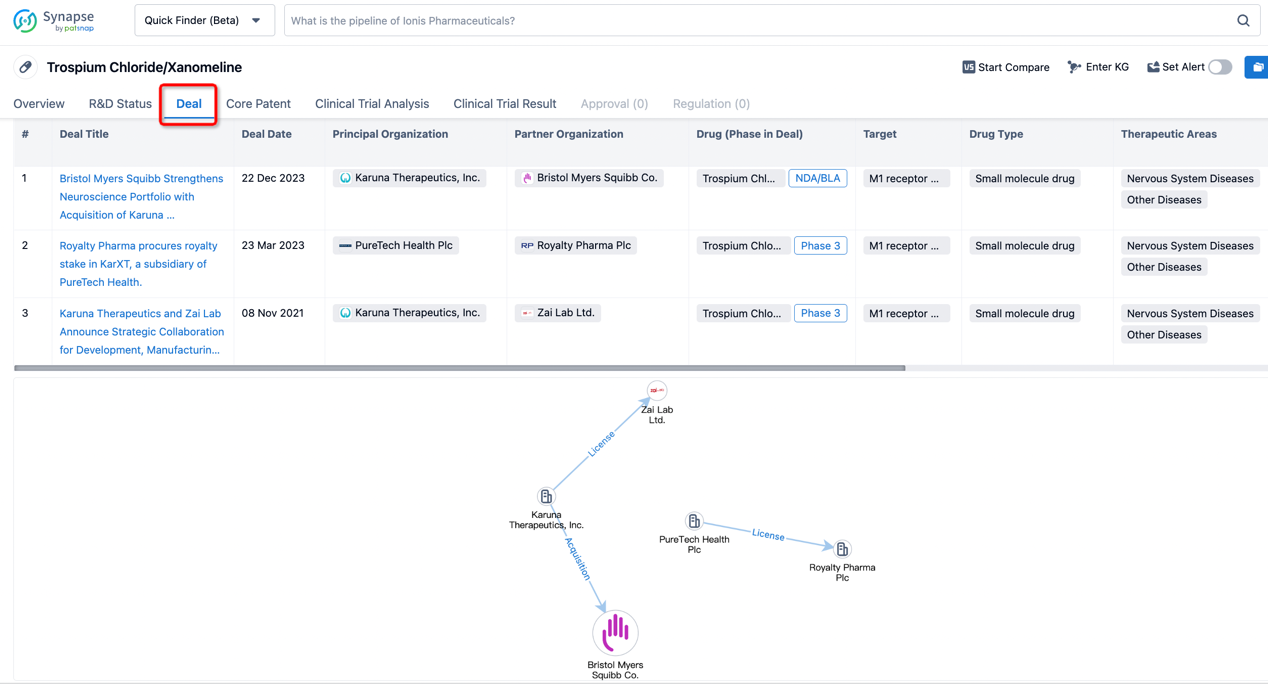
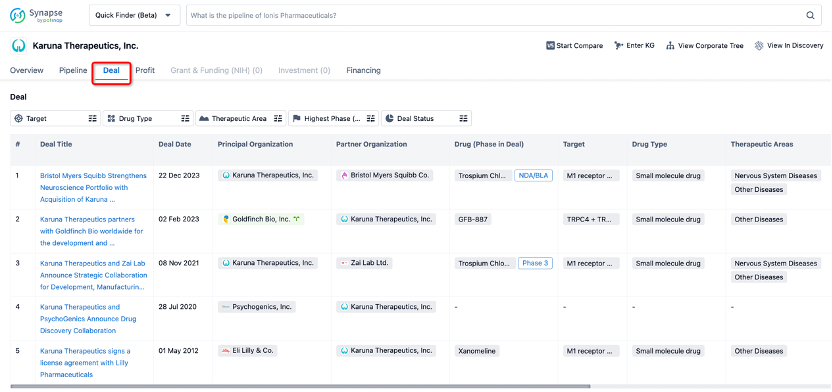
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to explore new pharmaceutical funding transactions!
Reference
1. Yu B, Jiang T, Liu D. BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol. 2020 Sep 17;13(1):125. doi: 10.1186/s13045-020-00962-7. PMID: 32943087; PMCID: PMC7499842.
2. van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018 Jan 4;131(1):13-29. doi: 10.1182/blood-2017-06-740944. Epub 2017 Nov 8. PMID: 29118010.
3. Corren J, Ziegler SF. TSLP: from allergy to cancer. Nat Immunol. 2019 Dec;20(12):1603-1609. doi: 10.1038/s41590-019-0524-9. Epub 2019 Nov 19. PMID: 31745338.
4. Zhou D, Wang Y, Chen C, Li Z, Xu K, Zhao K. Targeting GPRC5D for multiple myeloma therapy. J Hematol Oncol. 2024 Sep 28;17(1):88. doi: 10.1186/s13045-024-01611-z. PMID: 39342286; PMCID: PMC11439263.
5. Pavlasova G, Mraz M. The regulation and function of CD20: an "enigma" of B-cell biology and targeted therapy. Haematologica. 2020 Jun;105(6):1494-1506. doi: 10.3324/haematol.2019.243543. PMID: 32482755; PMCID: PMC7271567.