Ionis reports positive early results from Phase 3 trial of Olezarsen for familial chylomicronemia syndrome patients
Ionis Pharmaceuticals, Inc. has released encouraging preliminary findings from the Phase 3 Balance experiment of olezarsen on individuals suffering from familial chylomicronemia syndrome. The exploration successfully reached its main effectiveness goal by achieving a statistically significant decrease in triglyceride concentrations with monthly 80 mg doses of olezarsen for six months compared to the placebo; furthermore, the reduction in triglyceride levels continued to enhance at 12 months.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Administration of olezarsen at a dosage of 80 mg led to more than a 75% decrease in apoC-III. This is a protein generated in the liver that controls TG or triglyceride metabolism in the bloodstream. Besides the 80 mg monthly dosage, a 50 mg monthly dosage was also examined in the study. Both dosages of olezarsen manifested a dose-dependent impact, leading to a considerable decrease in pancreatitis instances. However, the 50 mg dosage did not meet the statistical significance criteria on the first endpoint of triglyceride reduction at a six-month check.
Ionis is planning to submit a New Drug Application to the U.S. FDA in early 2024, as well as to EU regulatory authorities. If given the green light, olezarsen will be the first-ever treatment for FCS in the U.S, a severe genetic disorder that could potentially trigger fatal pancreatitis attacks.
In 2023, the FDA awarded olezarsen a Fast Track designation for FCS treatment with the goal to speed up their scrutiny of innovative drugs that show promise to fill medical gaps. Ionis plans to share the Phase 3 data on olezarsen FCS at an upcoming medical conference.
Sam Tsimikas, M.D., the senior vice president of global cardiovascular development at Ionis stated, "Due to the absence of FDA-approved treatments, individuals with FCS suffer from severe abdominal discomfort and are required to follow a strict diet that includes less than 20 grams of fat per day." He added, "This research indicates that FCS patients who were administered olezarsen, along with getting background treatment and a low-fat diet, were at a significantly reduced risk of subsequent acute pancreatitis attacks".
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
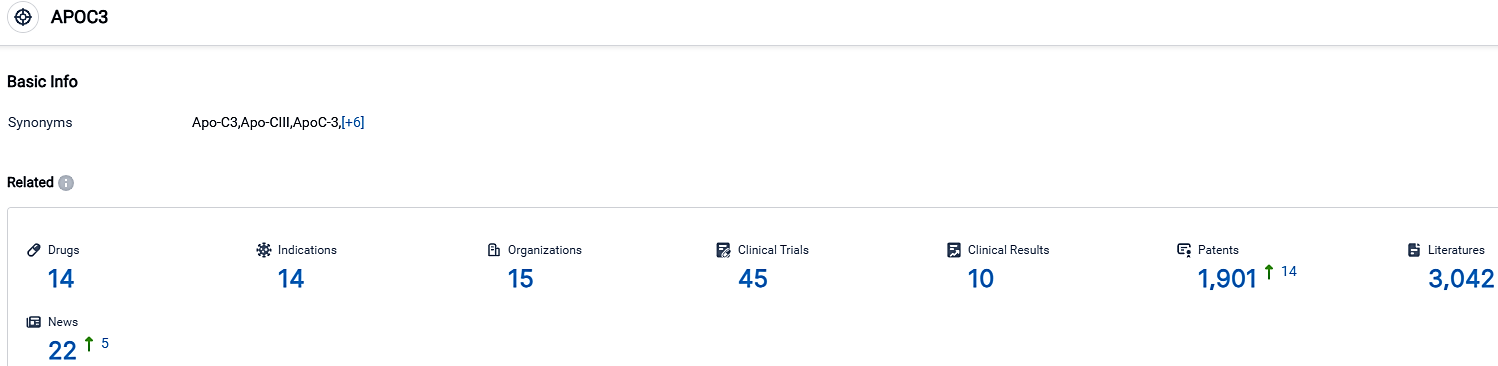
According to the data provided by the Synapse Database, As of October 3, 2023, there are 14 investigational drugs for the APOC3 target, including 14 indications,15 R&D institutions involved, with related clinical trials reaching 45,and as many as 1901 patents.
Olezarsen is formulated to suppress the biosynthesis of apoC-III, a liver-produced protein responsible for controlling TG metabolism in one's bloodstream. The FDA conferred Fast Track status to olezarsen for FCS therapy in the first quarter of 2023. Apart from FCS, Ionis is also assessing the efficacy of olezarsen in treating serious hypertriglyceridemia, as part of their Phase 3 clinical studies.