Iptacopan Reduces Proteinuria in IgA Nephropathy: Novartis Reports Significant Results
Novartis disclosed findings from a predetermined interim evaluation of the Phase III APPLAUSE-IgAN trial, examining Fabhalta (iptacopan), a Factor B inhibitor targeting the alternative complement pathway, for treating IgA nephropathy (IgAN). The data revealed that, at nine months, individuals receiving Fabhalta showed a 38.3% decrease in proteinuria, in comparison to those given placebo alongside standard care.
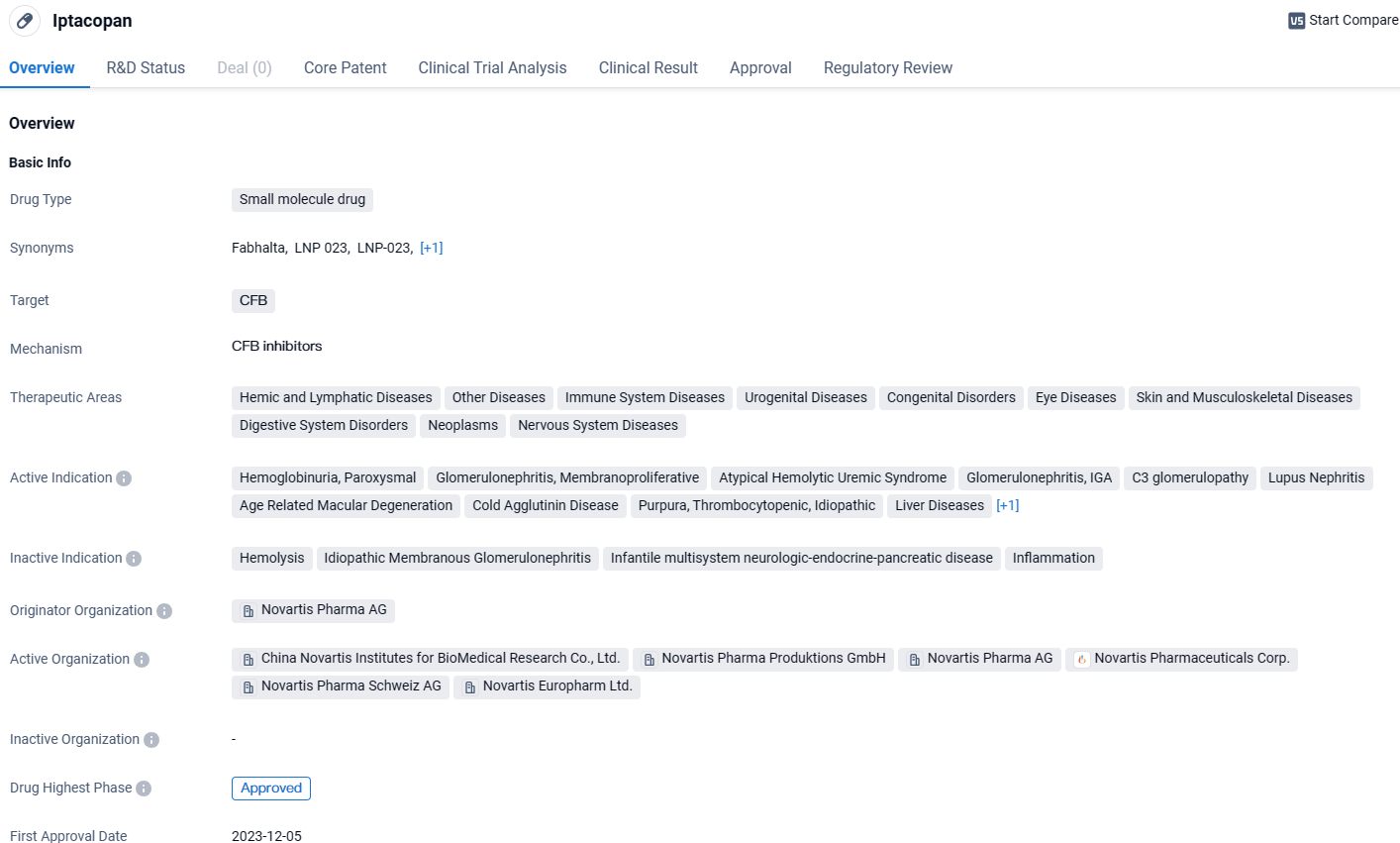
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Proteinuria reduction has become a valuable indicator linked with the advancement towards renal failure and is often utilized as a clinical endpoint in IgAN clinical studies to expedite regulatory approvals. It has been observed that Fabhalta is well-received with a strong safety profile aligning with earlier findings. These outcomes were introduced in a session dedicated to late-breaking clinical trials at the World Congress of Nephrology held in Buenos Aires, Argentina.
"In cases of IgAN, the immune system’s alternative complement pathway may overactivate within the kidneys, triggering an inflammatory reaction. This can cause ongoing kidney deterioration and a continuous decline in kidney functions. Alongside this, the adverse effects of previously available treatments for IgAN notably alter patients' quality of life," explained Professor Dana Rizk, a key investigator and member of the APPLAUSE-IgAN Steering Committee, also affiliated with the UAB Division of Nephrology. “Fabhalta represents the inaugural therapy aimed specifically at the alternative complement pathway for managing IgAN.”
The interim analysis, as initially planned, encompassed 250 patients for assessing efficacy and 443 patients for safety evaluations. The APPLAUSE-IgAN trial is proceeding under a double-blind setting, hence only a segment of the interim results are currently available. The submission for a fast-track endorsement by the FDA has been approved and given precedence. The chief measure for Fabhalta involves analyzing its effectiveness in decelerating IgAN progression by evaluating the annual total estimated glomerular filtration rate slope over a 24-month period, with final results anticipated by 2025.
"Given that IgAN develops gradually over several years, patient requirements may shift, implying that varying treatments might be more suitable at different stages," stated David Soergel, M.D., Global Head of the Cardiovascular, Renal, and Metabolism Development Unit at Novartis. "Our renal portfolio includes therapies featuring diverse mechanisms, potentially enabling personalized treatment based on individual clinical profiles."
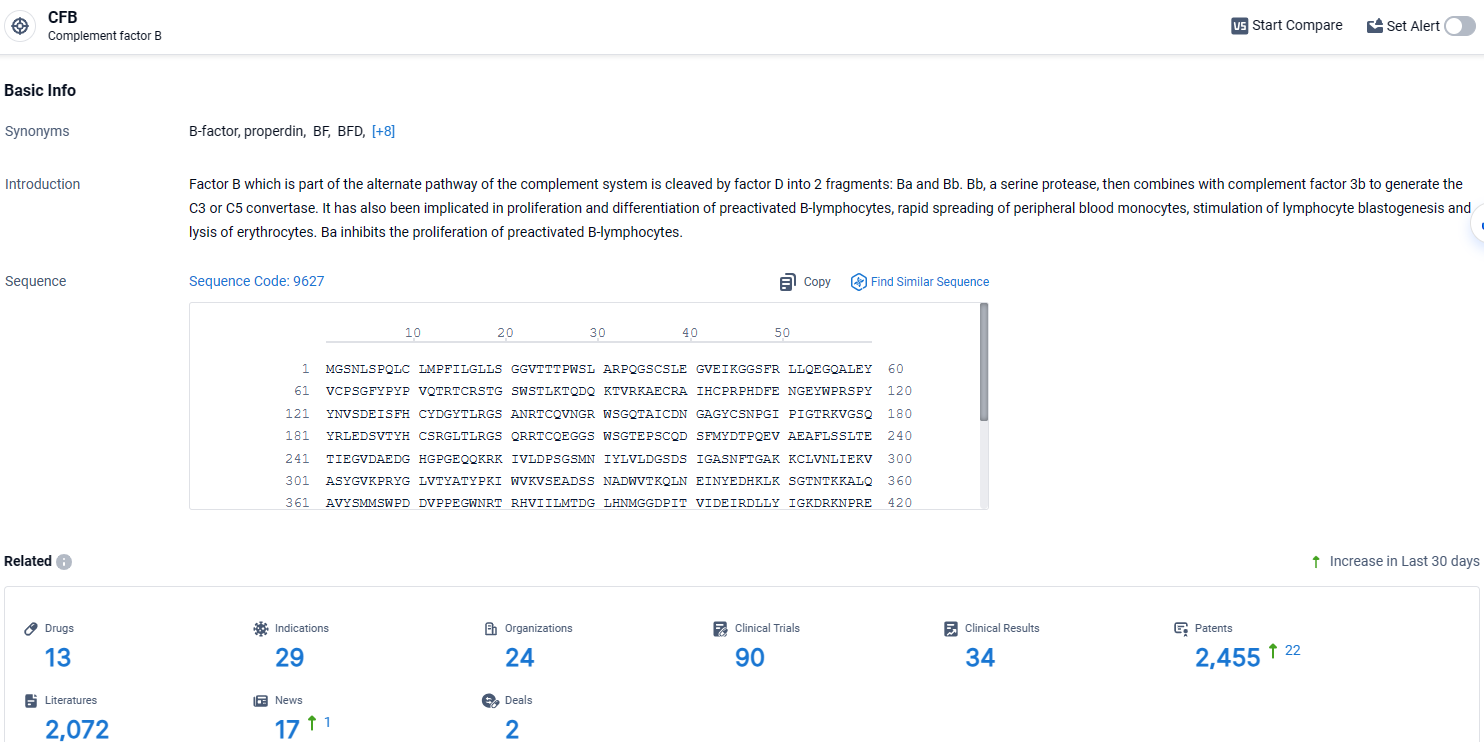
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 17, 2024, there are 13 investigational drugs for the CFB target, including 29 indications, 24 R&D institutions involved, with related clinical trials reaching 90, and as many as 2455 patents.
Iptacopan is a promising small molecule drug that targets the CFB protein and has shown potential in treating a wide range of diseases. Its highest phase of approval globally, upcoming approval in the United States, and regulatory designations highlight its potential to address unmet medical needs and provide significant clinical benefits to patients.