Is Avapritinib approved by the FDA?
Yes, Avapritinib, marketed under the brand name Ayvakit, is FDA approved. The U.S. Food and Drug Administration (FDA) approved Avapritinib on January 9, 2020, for the treatment of unresectable or metastatic gastrointestinal stromal tumors (GIST) harboring a PDGFRA exon 18 mutation, including PDGFRA D842V mutations.
What is Avapritinib?
Avapritinib is a medication used to treat certain types of cancers and rare blood disorders. It belongs to the class of drugs known as multikinase inhibitors. The drug is administered orally in tablet form and is available in various dosages, including 25 mg, 50 mg, 100 mg, 200 mg, and 300 mg.
Uses of Avapritinib
- Gastrointestinal Stromal Tumors (GIST):
- Indication: Avapritinib is used to treat adults with unresectable or metastatic GIST with a PDGFRA exon 18 mutation.
- Dosage: The usual dose for GIST is 300 mg orally once a day until disease progression or unacceptable toxicity occurs.
- Systemic Mastocytosis:
- Advanced Systemic Mastocytosis (AdvSM): Includes aggressive systemic mastocytosis, systemic mastocytosis with an associated hematological neoplasm, and mast cell leukemia.
- Dosage: 200 mg orally once a day.
- Indolent Systemic Mastocytosis (ISM):
- Dosage: 25 mg orally once a day.
- Advanced Systemic Mastocytosis (AdvSM): Includes aggressive systemic mastocytosis, systemic mastocytosis with an associated hematological neoplasm, and mast cell leukemia.
Important Precautions
- Genetic Marker: Patients must have a specific genetic marker (an abnormal PDGFRA gene) for the medication to be effective in treating GIST.
- Blood Counts: Not recommended for AdvSM or ISM patients with platelet counts below 50 x 10^9/L.
- Pregnancy and Birth Control: Both men and women using Avapritinib should use effective birth control, as the drug can cause harm to an unborn baby or result in birth defects.
Side Effects
Avapritinib can cause several side effects, some of which can be serious. Common side effects include:
- Nausea, vomiting, and loss of appetite
- Stomach pain, diarrhea, or constipation
- Fluid retention and swelling
- Dizziness, weakness, and fatigue
- Muscle weakness, watery eyes, rash, and hair color changes
Serious side effects that require immediate medical attention include severe headaches, vision problems, changes in mood or behavior, speech difficulties, confusion, hallucinations, severe drowsiness, trouble sleeping, and significant weakness on one side of the body.
Storage and Administration
Avapritinib should be taken on an empty stomach, at least 1 hour before or 2 hours after a meal. It should be stored at room temperature, away from moisture and heat.
Conclusion
Avapritinib (Ayvakit) is an FDA-approved medication effective in treating certain types of cancer and systemic mastocytosis. Approved on January 9, 2020, it offers targeted treatment for patients with specific genetic markers, providing a valuable option for managing these challenging conditions. Patients should consult their healthcare provider to determine if Avapritinib is suitable for their treatment regimen.
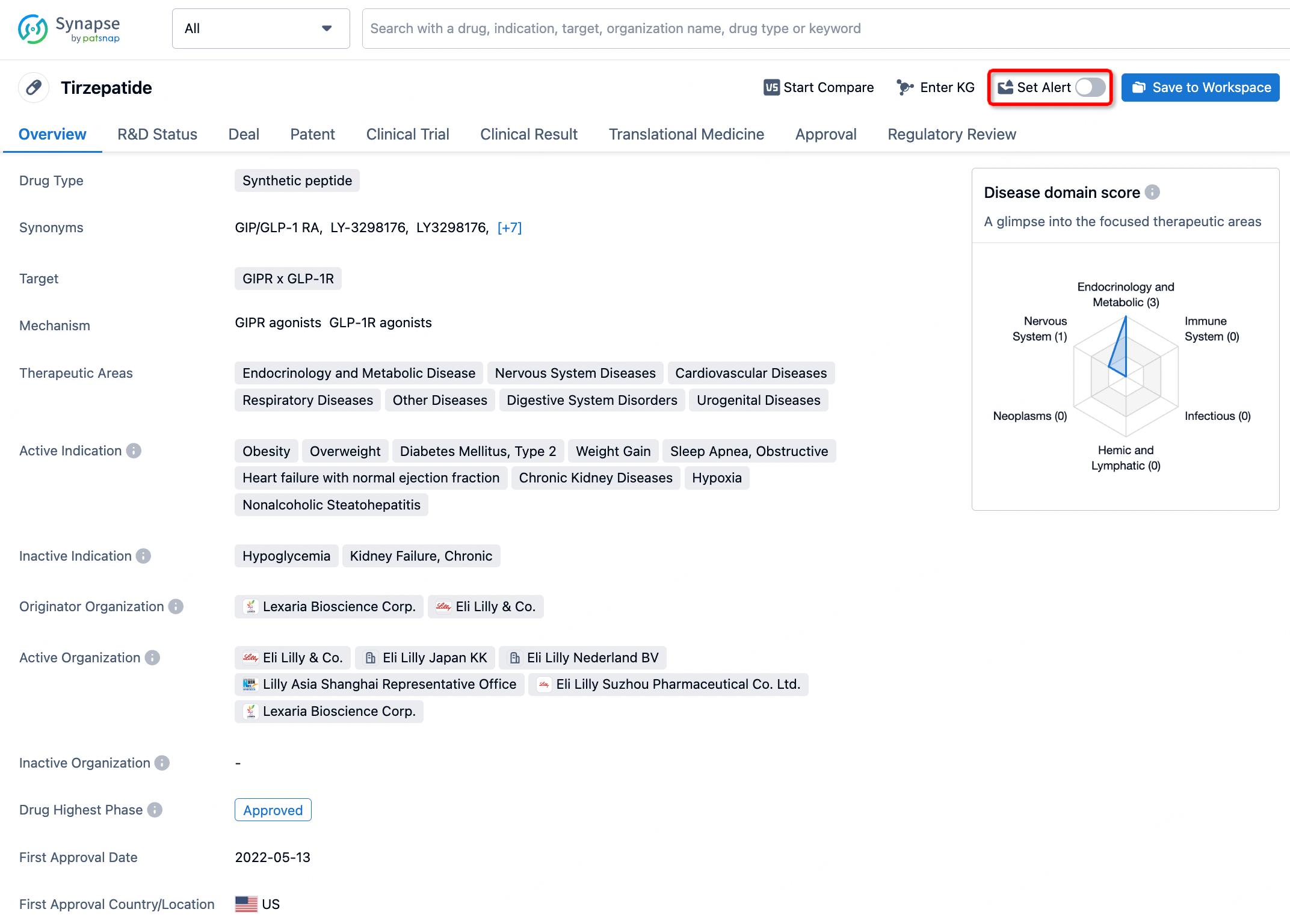
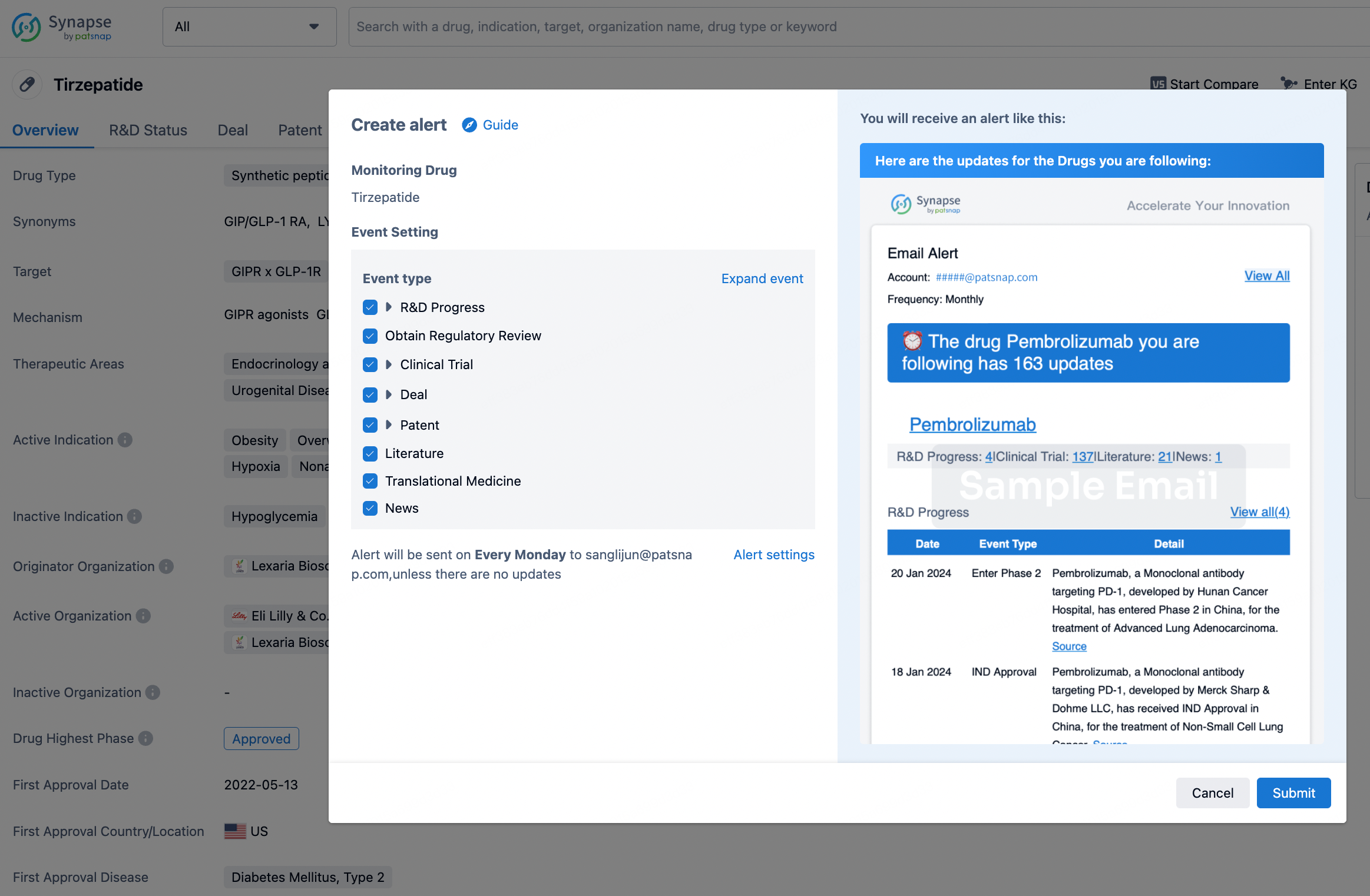
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!