Is Cabtreo approved by the FDA?
Cabtreo, a combination of clindamycin phosphate, adapalene, and benzoyl peroxide, was FDA-approved on October 20, 2023. This approval makes Cabtreo the first triple combination treatment approved for acne.
What is Cabtreo?
Cabtreo is a topical gel that combines three active ingredients to treat acne in adults and children aged 12 and older:
- Clindamycin phosphate: An antibiotic that targets Cutibacterium acnes, a bacteria associated with acne.
- Adapalene: A synthetic retinoid that helps restore skin tone and texture and reduces inflammation.
- Benzoyl peroxide: An oxidizing agent with bactericidal and keratolytic effects.
Cabtreo is designed for use on the skin only and should not be used in the mouth, eyes, or vagina.
How to Use Cabtreo
- Application: Apply once a day, at the same time each day. Cleanse your face with a mild cleanser, rinse with warm water, and pat dry before application.
- Dosage: Dispense a pea-sized amount and dot it onto six areas of your face (chin, left/right cheek, nose, left/right forehead). Spread the gel gently over your entire face, avoiding eyes, mouth, and nostrils.
- Post-application: Wash your hands immediately after applying the gel.
Warnings and Precautions
- Hypersensitivity: Do not use if you are allergic to clindamycin, adapalene, benzoyl peroxide, or any components of the formulation. Discontinue use if a hypersensitivity reaction occurs.
- Colon Inflammation: Avoid use if you have a history of colitis or severe diarrhea with past antibiotic use, as clindamycin can cause severe colitis.
- Photosensitivity: Cabtreo may increase skin sensitivity to sunlight. Avoid excessive sun exposure and use sunscreen and protective clothing.
- Skin Irritation: May cause redness, scaling, dryness, stinging, or burning. Discontinue use if irritation persists.
Pregnancy and Breastfeeding
- Pregnancy: The effects of Cabtreo on an unborn baby are unknown. Discuss the risks and benefits with your doctor if you are pregnant or planning to become pregnant.
- Breastfeeding: It is unknown if Cabtreo passes into breast milk. If used while breastfeeding, apply to the smallest area for the shortest time and avoid application near the nipple.
Side Effects
Common side effects include skin reactions such as:
- Redness
- Scaling
- Dryness
- Stinging
- Burning
- Itching
- Peeling
- Swelling
Serious side effects requiring immediate medical attention:
- Severe allergic reactions (hives, swelling, trouble breathing)
- Colitis (severe stomach cramps, watery or bloody diarrhea)
Drug Interactions
- Erythromycin: Do not use Cabtreo with products containing erythromycin.
- Neuromuscular Blocking Agents: Use with caution.
- Other Skin Products: May increase skin irritation.
Storage
- Temperature: Store at room temperature (below 77°F or 25°C).
- Conditions: Do not freeze. Keep away from heat and store the pump upright.
- Expiration: Discard any expired product and keep out of reach of children.
Conclusion
Cabtreo, approved by the FDA on October 20, 2023, offers a comprehensive treatment for acne with its triple combination formula. If you are considering Cabtreo, follow your healthcare provider's instructions and be mindful of potential side effects and precautions.
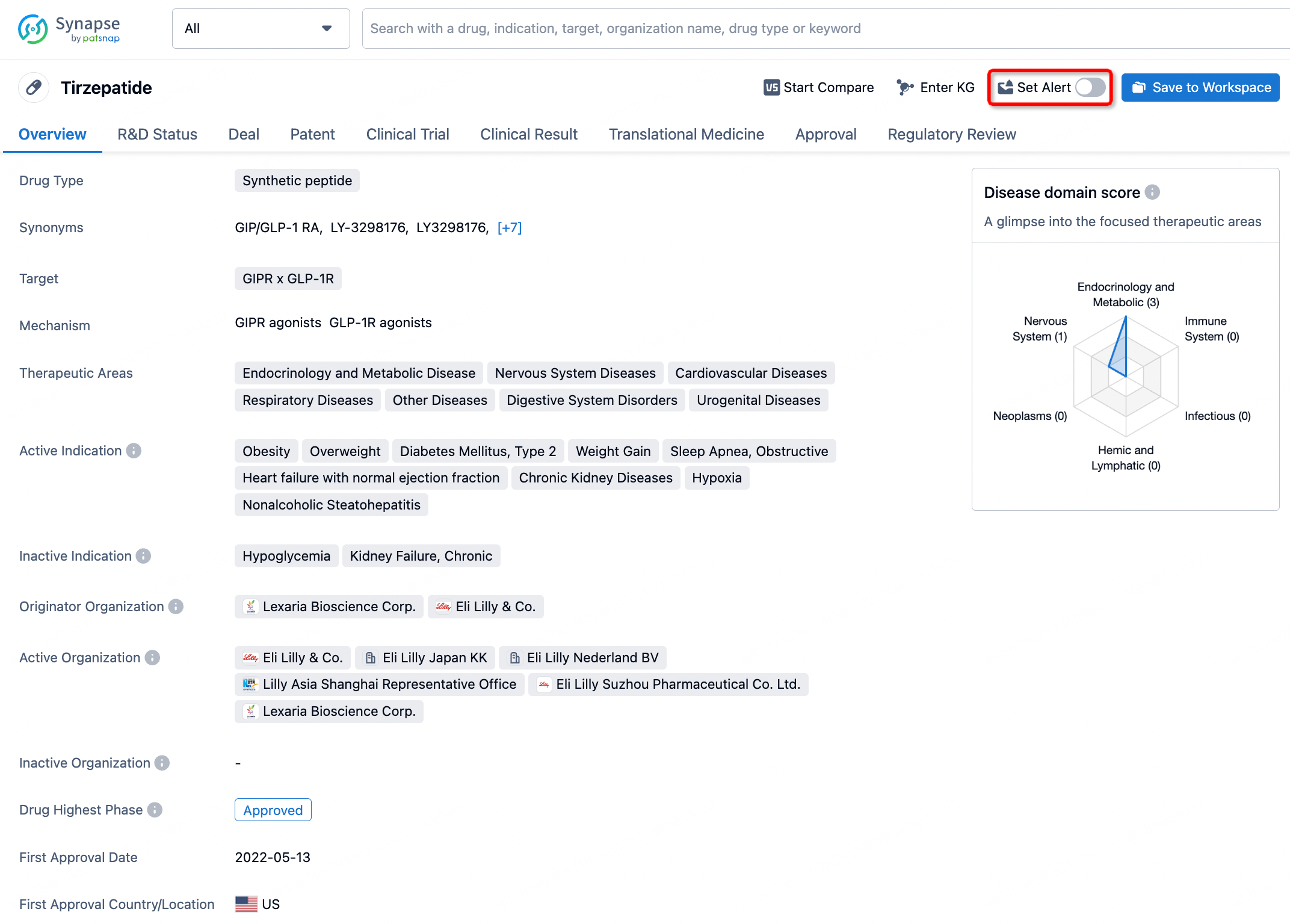
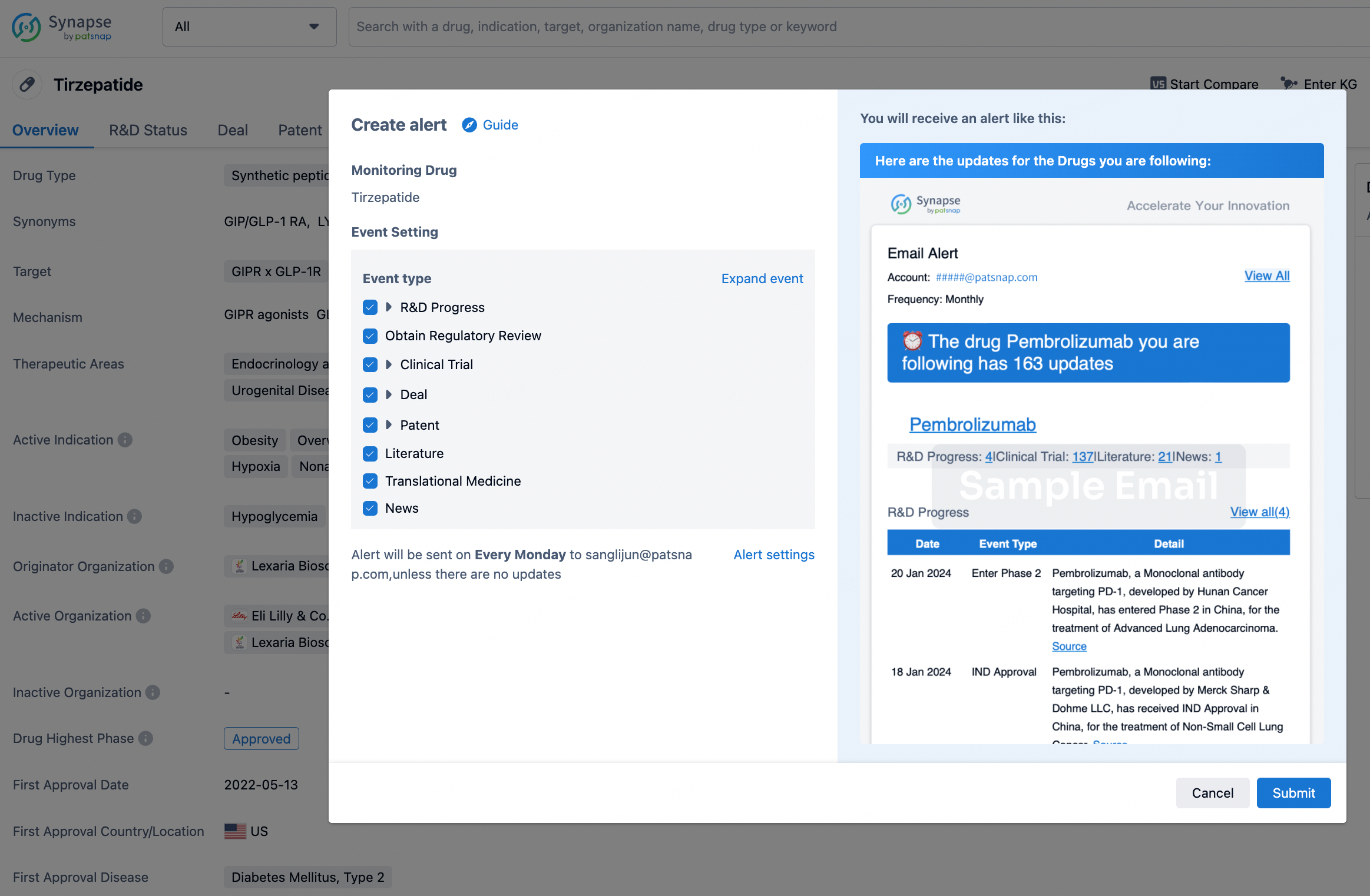
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!