Is Crizanlizumab approved by the FDA?
Yes, Crizanlizumab, marketed under the brand name Adakveo, is FDA approved. The U.S. Food and Drug Administration (FDA) approved Crizanlizumab on November 15, 2019. This approval was specifically for its use in reducing the frequency of vaso-occlusive crises (painful episodes) in patients with sickle cell disease.
What is Crizanlizumab?
Crizanlizumab is a monoclonal antibody used to treat sickle cell disease by reducing the occurrence of vaso-occlusive crises (VOCs). These crises are painful episodes that occur when the sickled red blood cells block blood flow to different parts of the body. Crizanlizumab works by binding to a protein called P-selectin on the surface of blood vessels and certain blood cells, preventing these cells from sticking to each other and the vessel walls, thus reducing the blockage and associated pain.
Indications and Usage
Crizanlizumab is indicated for the treatment of patients with sickle cell disease to reduce the frequency of vaso-occlusive crises. It is approved for use in adults and children aged 16 years and older.
Dosage and Administration
Crizanlizumab is administered as an intravenous (IV) infusion. The typical dosing schedule is:
- Initial Dose: 5 mg/kg given by IV infusion every 2 weeks for the first 2 doses.
- Maintenance Dose: 5 mg/kg given by IV infusion every 4 weeks thereafter.
Each infusion takes about 30 minutes to complete. The dosage is calculated based on the patient’s actual body weight and can be given with or without hydroxyurea, another medication commonly used to treat sickle cell disease.
Side Effects
Common Side Effects
- Stomach pain or tenderness
- Nausea
- Fever
- Joint pain
- Back pain
Serious Side Effects
Some side effects may occur within 24 hours of receiving Crizanlizumab, including:
- Dizziness
- Nausea
- Fatigue
- Itching
- Chills
- Sweating
- Fever
- Wheezing
- Shortness of breath
Warnings and Precautions
- Allergic Reactions: Immediate medical attention is required if signs of an allergic reaction occur, such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat.
- Pregnancy and Breastfeeding: It is essential to discuss with a healthcare provider if you are pregnant, planning to become pregnant, or breastfeeding. Sickle cell disease during pregnancy may increase the risk of premature birth or low birth weight, so the benefits of treating sickle cell disease may outweigh potential risks to the baby.
Interactions
Patients should inform their healthcare provider about all medications they are taking, including prescription drugs, over-the-counter medicines, vitamins, and herbal supplements, as these can affect the efficacy and safety of Crizanlizumab.
Storage
Crizanlizumab should be stored according to the healthcare provider's instructions, typically under refrigeration until it is ready to be used.
Conclusion
Crizanlizumab (Adakveo) is an FDA-approved treatment that offers a new option for patients with sickle cell disease to manage and reduce the frequency of vaso-occlusive crises. Approved in November 2019, it represents a significant advancement in the management of this chronic and painful condition. Patients should work closely with their healthcare providers to understand the benefits and risks associated with this treatment.
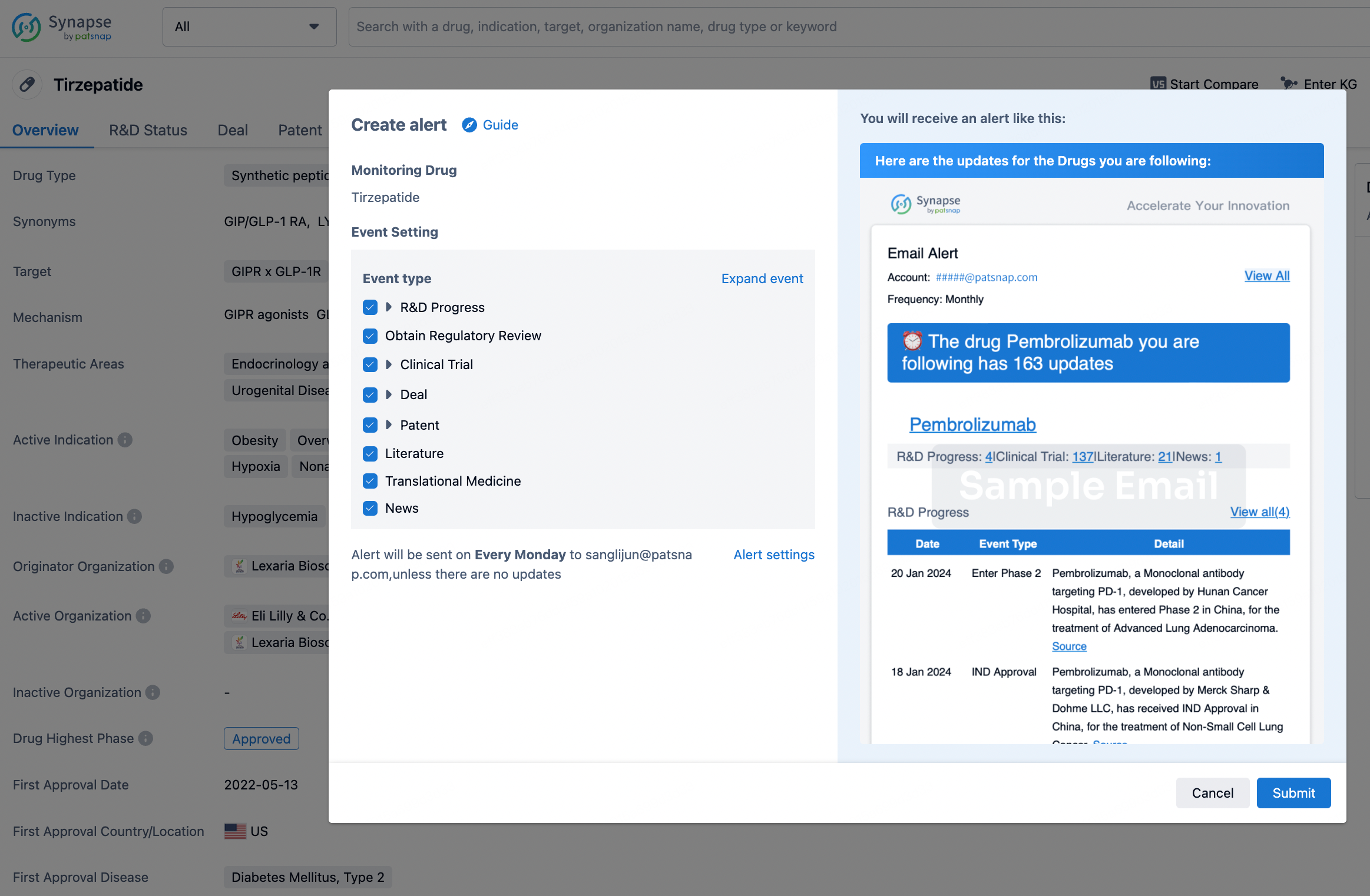
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!