Is Detectnet approved by the FDA?
Yes, Detectnet (copper Cu 64 dotatate) is an FDA-approved diagnostic radiopharmaceutical used in PET scans to locate somatostatin receptor-positive neuroendocrine tumors. Approved on September 3, 2020, it provides a critical tool for the diagnosis and management of NETs.
What is Detectnet?
Detectnet is a diagnostic radiopharmaceutical used in positron emission tomography (PET) scans for the localization of somatostatin receptor-positive neuroendocrine tumors (NETs). It involves the injection of copper Cu 64 dotatate, which is a radioactive agent that helps visualize certain tumors during PET scans.
How Detectnet Works
Detectnet works by targeting somatostatin receptors that are often overexpressed in neuroendocrine tumors. When injected into the body, the radioactive copper Cu 64 dotatate binds to these receptors, allowing for clear imaging of the tumors during a PET scan.
Administration and Dosage
- Administration: Detectnet is administered as an intravenous injection by a healthcare professional trained in nuclear medicine, just before the patient undergoes a PET scan.
- Preparation: Patients are advised to stay well-hydrated before the PET scan and are encouraged to urinate frequently for at least an hour after the scan to help eliminate the radioactive material from their bodies.
Potential Side Effects
Common Side Effects:
- Feeling of warmth
- Nausea
- Redness of the face, neck, arms, and occasionally upper chest
- Vomiting
Serious Side Effects:
- Chest tightness
- Cough
- Difficulty swallowing
- Dizziness
- Fast heartbeat
- Hives, itching, skin rash
- Swelling of face, eyelids, lips, tongue, throat, hands, legs, feet, or sex organs
- Trouble breathing
- Unusual tiredness or weakness
It is important for patients to report any severe or unusual symptoms to their doctor immediately.
Warnings and Precautions
- Allergies: Patients should inform their doctor of any known allergies to medications, foods, dyes, preservatives, or animals.
- Pediatric Use: The safety and efficacy of Detectnet in pediatric patients have not been established.
- Geriatric Use: No specific geriatric-related problems have been identified, but caution is advised due to potential heart, kidney, or liver issues in elderly patients.
- Breastfeeding: Women should weigh the potential risks and benefits before using this medication during breastfeeding.
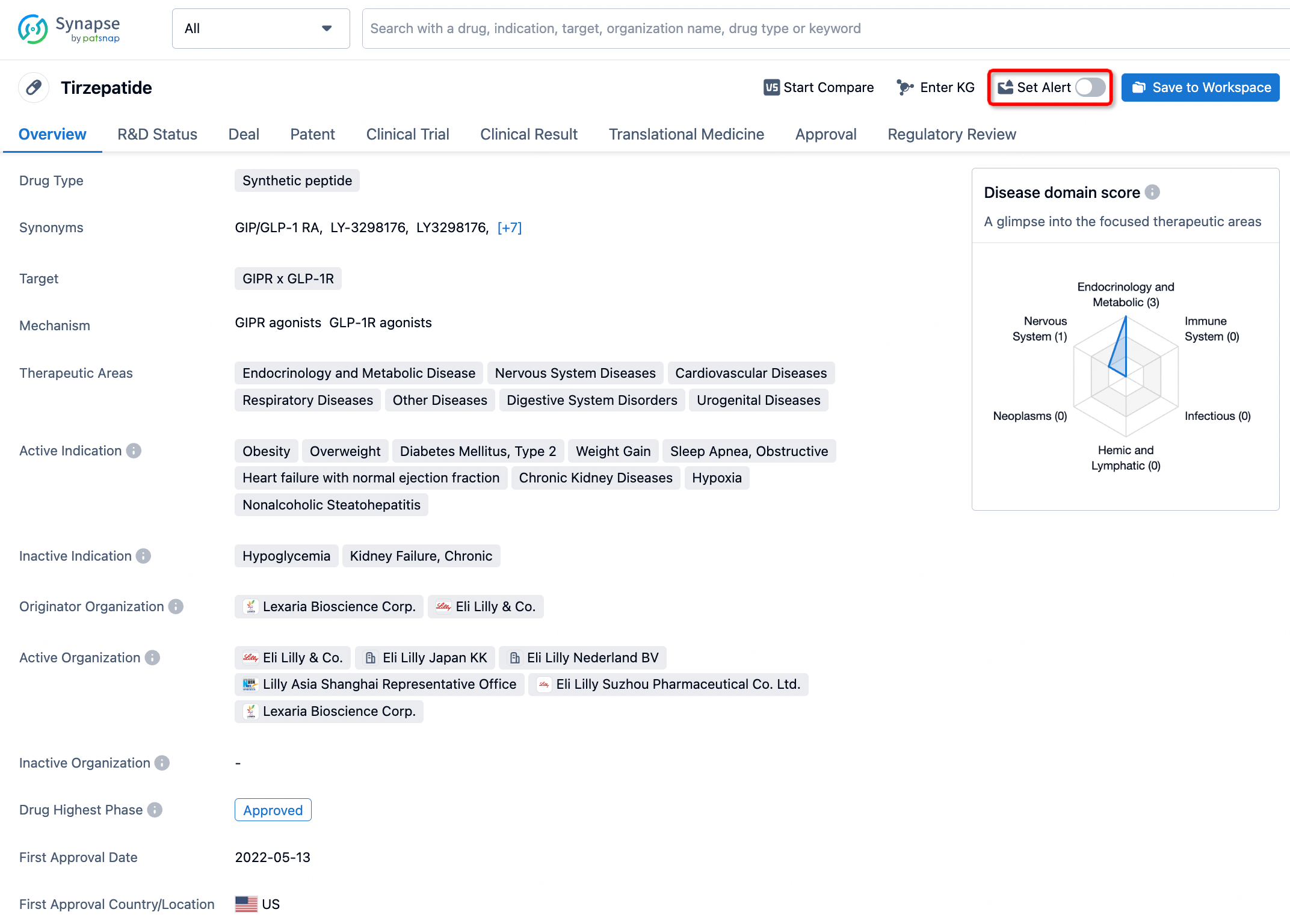
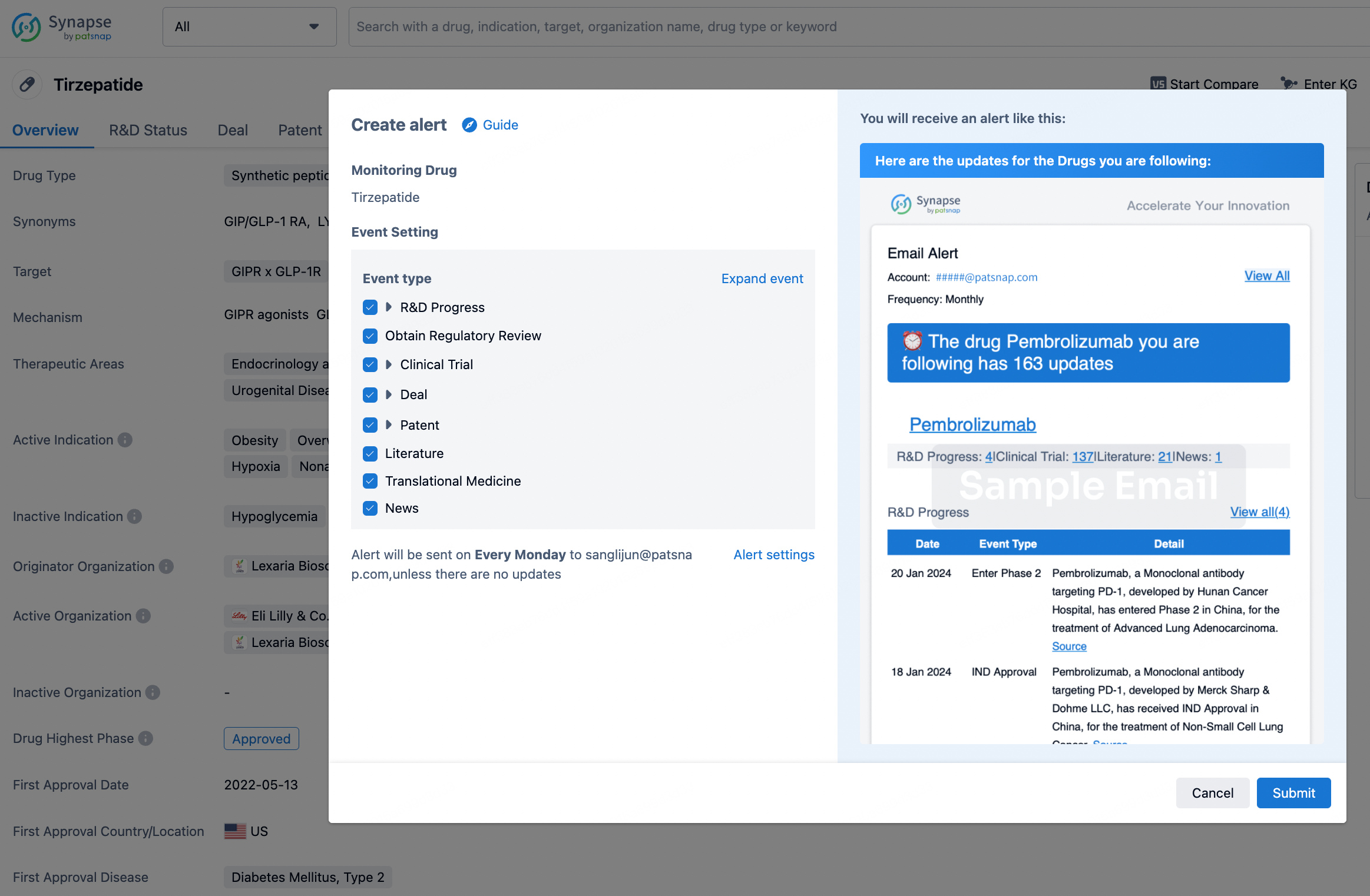
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!