Is Ibrexafungerp approved by the FDA?
Ibrexafungerp, marketed under the brand name Brexafemme, is an antifungal medication used to treat and reduce the recurrence of vaginal yeast infections (vulvovaginal candidiasis) in women and girls who have begun menstruating. Brexafemme (ibrexafungerp) was approved by the U.S. Food and Drug Administration (FDA) on June 1, 2021. This approval marks a significant development as Brexafemme is the first in a new class of antifungal agents known as triterpenoids.
Indications and Usage
Brexafemme is indicated for:
- The treatment of adult and postmenarchal pediatric females with vulvovaginal candidiasis (VVC).
- The reduction in the incidence of recurrent vulvovaginal candidiasis (RVVC) in adult and postmenarchal pediatric females.
Administration
Brexafemme is available in oral tablet form with a dosage strength of 150 mg. The typical dosing regimen is:
- For treatment: 300 mg (two 150 mg tablets) taken twice in one day, approximately 12 hours apart.
- To prevent recurrences: 300 mg taken twice in one day, approximately 12 hours apart, repeated once a month for six months.
Patients can take Brexafemme with or without food.
Side Effects and Warnings
Common side effects of ibrexafungerp include:
- Headache
- Nausea
- Loose stools or diarrhea
- Stomach pain
- Vomiting
- Dizziness
Serious allergic reactions may occur, presenting as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat. In case of severe side effects, it is important to seek medical attention immediately.
Brexafemme is not recommended for use during pregnancy due to potential harm to the unborn baby. Effective birth control should be used during treatment and for at least four days after the last dose. Breastfeeding safety should be discussed with a healthcare provider.
Precautions
Before taking Brexafemme, patients should inform their healthcare provider if they have any known allergies to ibrexafungerp or any other medications. Additionally, patients should provide a complete list of current medications, including over-the-counter drugs and supplements, to avoid potential drug interactions.
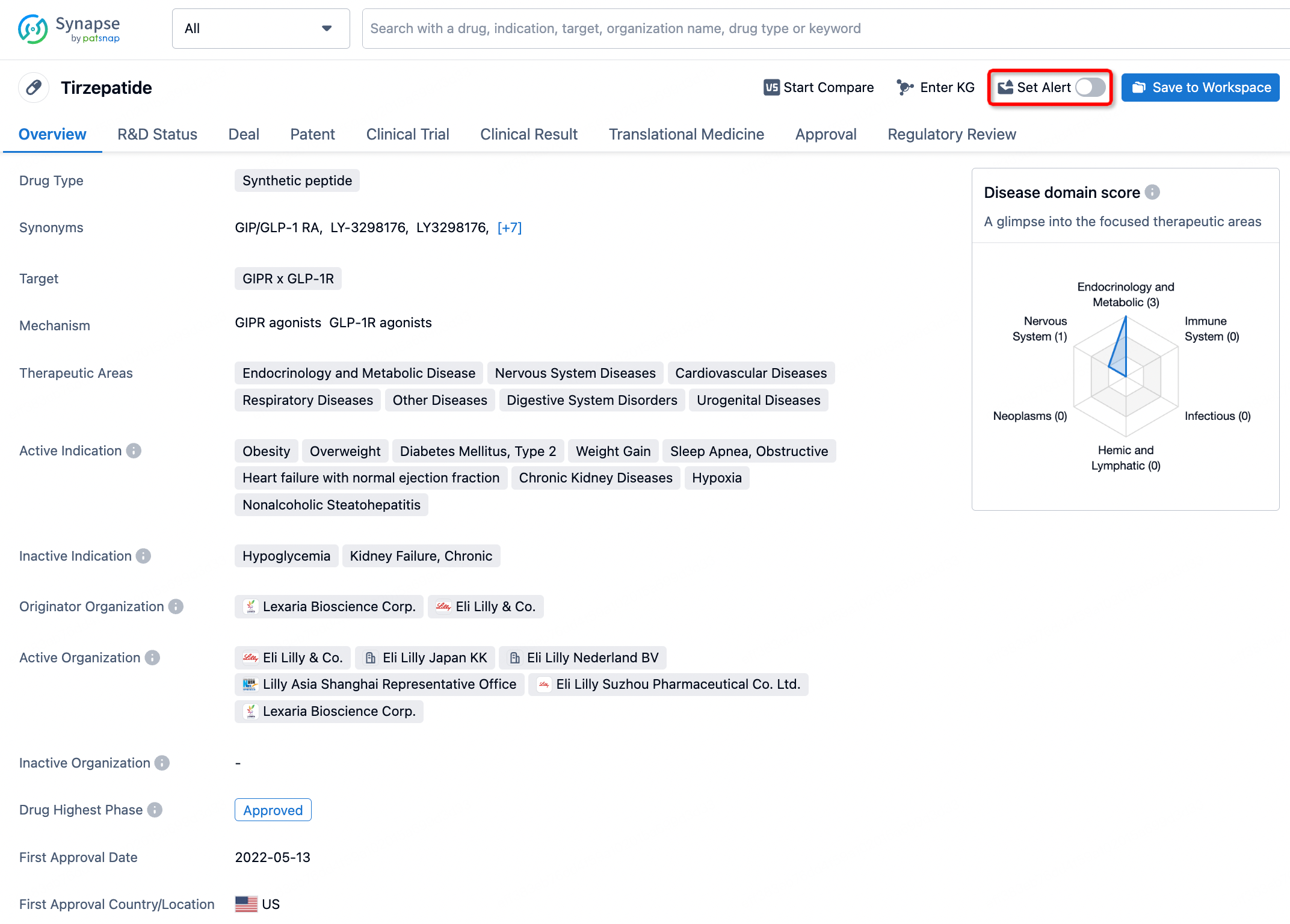
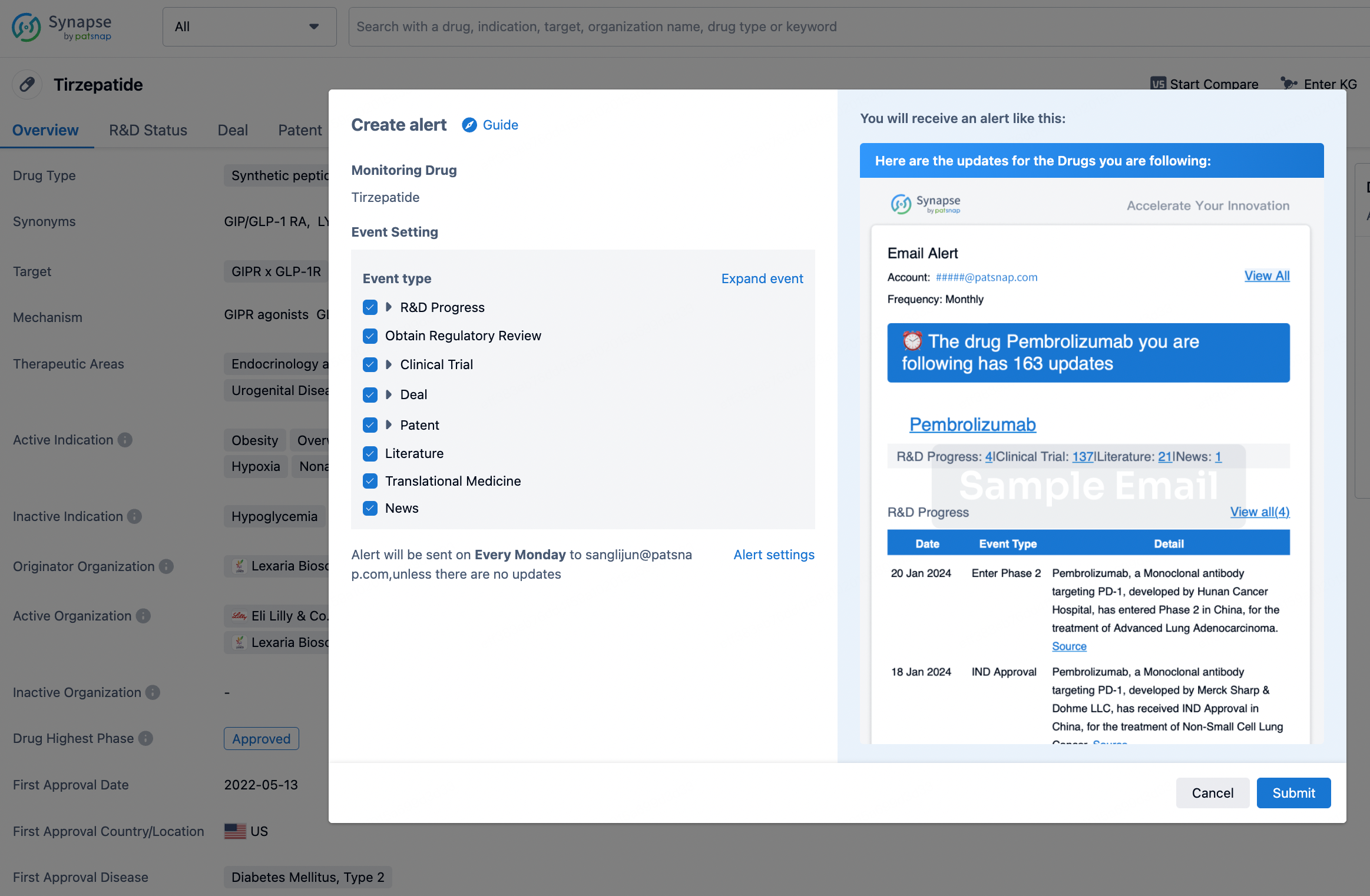
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!