Is Ponesimod approved by the FDA?
Ponesimod, marketed under the brand names Ponvory and Ponvory Starter Pack, is an oral medication used to treat relapsing forms of multiple sclerosis (MS) in adults. Ponvory (ponesimod) was approved by the U.S. Food and Drug Administration (FDA) on March 18, 2021. This approval provided a new treatment option for individuals with relapsing forms of multiple sclerosis, offering a novel mechanism of action aimed at improving patient outcomes.
Mechanism of Action
Ponesimod belongs to a class of drugs known as selective immunosuppressants. It works by selectively modulating the activity of the immune system to reduce the frequency of MS relapses and slow the progression of physical disability. Ponesimod achieves this by binding to the sphingosine-1-phosphate receptor 1 (S1P1), which is involved in lymphocyte migration. By reducing the number of lymphocytes in the peripheral blood, ponesimod helps to limit the autoimmune attack on the central nervous system seen in MS.
Usage and Administration
Ponesimod is administered orally in tablet form, with a specific dosing regimen designed to gradually increase the dose over the first 14 days of treatment:
- Initial Titration (Days 1-14):
- Day 1-2: 2 mg once daily
- Day 3-4: 3 mg once daily
- Day 5-6: 4 mg once daily
- Day 7: 5 mg once daily
- Day 8: 6 mg once daily
- Day 9: 7 mg once daily
- Day 10: 8 mg once daily
- Day 11: 9 mg once daily
- Day 12-14: 10 mg once daily
- Maintenance Dose:
- From Day 15 onwards: 20 mg once daily
Patients starting ponesimod should use the Ponvory Starter Pack, which contains tablets of different strengths for the titration period. The first dose should be administered under medical supervision, particularly for those with pre-existing cardiac conditions.
Side Effects and Warnings
Common Side Effects:
- Cold symptoms (stuffy nose, sore throat)
- High blood pressure
- Abnormal liver function tests
Serious Side Effects:
- Slow heartbeats, chest pain, shortness of breath
- Severe headache, vision changes, seizures
- Liver problems (nausea, dark urine, jaundice)
- Skin changes indicating potential cancer
- Symptoms of infection (fever, neck stiffness)
Precautions:
- Ponesimod can cause a decrease in heart rate, especially with the first dose.
- Infections: Patients may be more susceptible to infections, even serious ones.
- Vaccination: Avoid live vaccines during and shortly after treatment.
- Pregnancy: Use effective contraception during and for one week after treatment.
- Sun Exposure: Increased risk of skin cancer; use protective measures against sunlight.
Conclusion
It offers a new option for patients to manage their MS, particularly those who have experienced multiple relapses. As with any medication, it is essential to follow the prescribed regimen and be aware of potential side effects and necessary precautions to ensure safe and effective use.
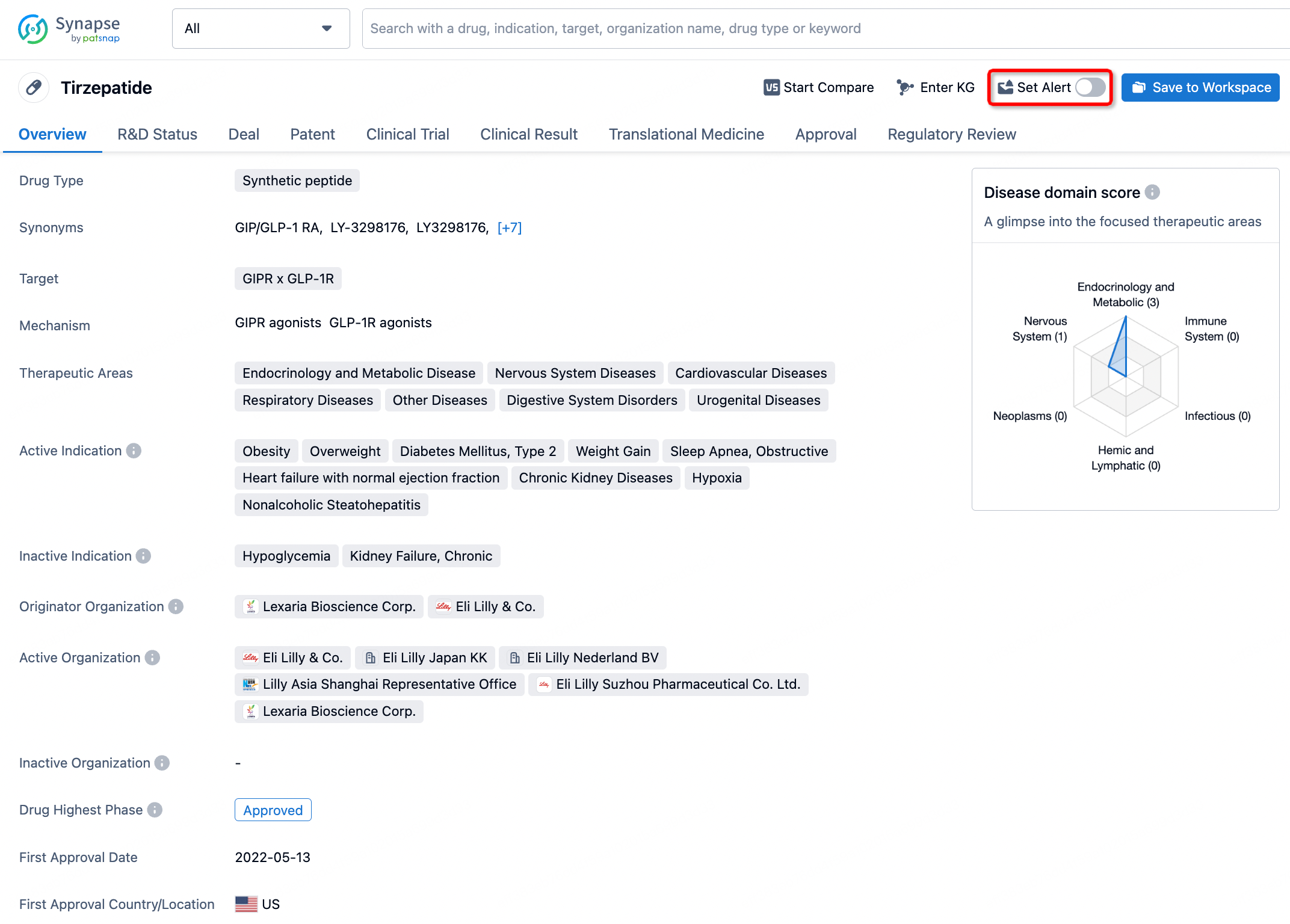
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!