Is Reblozyl approved by the FDA?
Yes, Reblozyl (generic name: luspatercept-aamt) is FDA approved. The U.S. Food and Drug Administration (FDA) approved Reblozyl on November 8, 2019, for the treatment of anemia in adult patients with beta thalassemia who require regular red blood cell (RBC) transfusions.
What is Reblozyl?
Reblozyl is a subcutaneous injection that comes in two dosage forms: 25 mg/vial and 75 mg/vial. It belongs to the drug class of miscellaneous erythropoiesis agents. Reblozyl is used to treat anemia by increasing the number of mature red blood cells (RBCs) in the bloodstream, thus reducing the need for blood transfusions. It is specifically indicated for:
- Beta Thalassemia: A rare inherited blood disorder causing ineffective red blood cell production, leading to anemia.
- Myelodysplastic Syndromes (MDS): A group of blood cancers where the body cannot produce healthy red blood cells, white blood cells, and platelets properly, resulting in anemia and the need for blood transfusions.
Indications and Usage
Reblozyl is FDA-approved to treat anemia in adult patients with:
- Beta Thalassemia: Patients requiring regular RBC transfusions.
- Very Low- to Intermediate-Risk Myelodysplastic Syndromes (MDS): Patients with anemia who have not previously used erythropoiesis-stimulating agents (ESA-naïve) and may require regular RBC transfusions.
- MDS with Ring Sideroblasts (MDS-RS) or Myelodysplastic/Myeloproliferative Neoplasm with Ring Sideroblasts and Thrombocytosis (MDS/MPN-RS-T): Patients who need regular RBC transfusions (two or more RBC units over eight weeks) and have not responded well to or cannot receive an erythropoiesis-stimulating agent (ESA).
Dosage and Administration
Reblozyl is administered as a subcutaneous injection, typically given once every three weeks. The dosage is based on the patient's weight, with initial dosing for anemia in beta thalassemia patients set at 1 mg/kg. If there is no reduction in RBC transfusions after two doses, the dose may be increased to a maximum of 1.25 mg/kg.
Side Effects
Common Side Effects
- Stomach pain, diarrhea, nausea
- Headache, dizziness
- Fatigue
- Cough, trouble breathing
- Muscle, bone, and joint pain
- Allergic reactions
Serious Side Effects
- Severe headache, blurred vision, pounding in the neck or ears
- Stroke symptoms: sudden numbness or weakness, severe headache, slurred speech, balance problems
- Blood clot symptoms: chest pain, sudden cough, wheezing, rapid breathing, coughing up blood; swelling, warmth, or redness in an arm or leg
Reblozyl may also cause fertility problems in females, potentially affecting their ability to become pregnant.
Warnings and Precautions
Patients should inform their doctor about all medical conditions, including:
- Spleen removal surgery or a history of enlarged spleen or liver
- History of stroke or blood clot
- High blood pressure, cholesterol, or diabetes
- Use of birth control pills or hormone replacement therapy
- History of extramedullary hematopoietic (EMH) masses
Pregnancy and Breastfeeding
Reblozyl may harm an unborn baby, so it is important for women who can become pregnant to use effective contraception during treatment and for at least three months after the last dose. Breastfeeding should be avoided while using Reblozyl and for at least three months after the last dose.
Conclusion
Reblozyl, approved by the FDA on November 8, 2019, provides a significant therapeutic option for managing anemia in patients with beta thalassemia and certain myelodysplastic syndromes. By increasing the production of mature red blood cells, it helps reduce the need for frequent blood transfusions, thereby improving the quality of life for these patients. As with any medication, patients should discuss potential side effects and interactions with their healthcare provider to ensure safe and effective use.
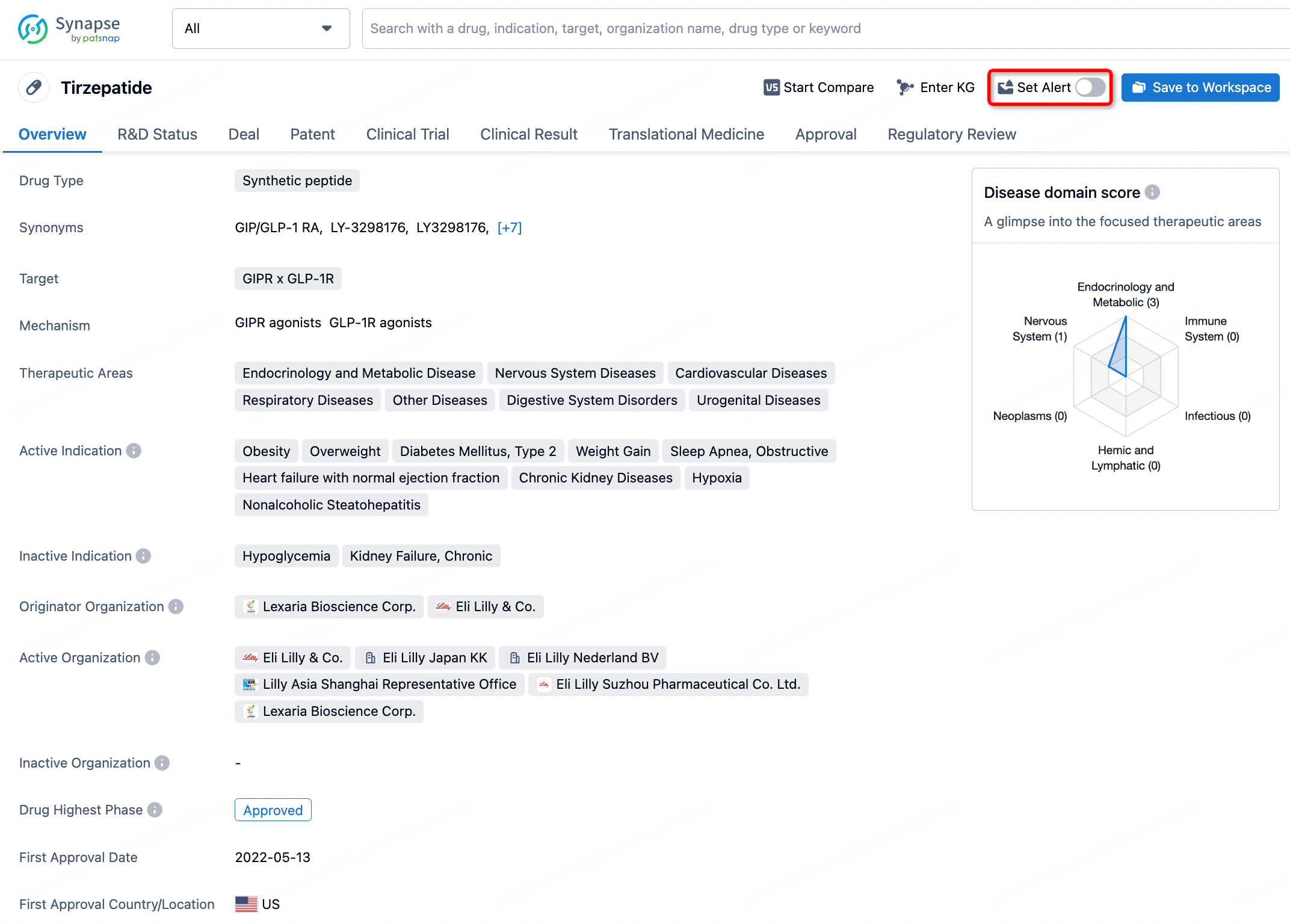
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!