Is Risdiplam approved by the FDA?
Yes, risdiplam, marketed under the brand name Evrysdi, is FDA approved. Its FDA approval on August 7, 2020, has provided new hope and convenience for individuals affected by SMA, improving the management and quality of life for patients with this condition.
What is Risdiplam?
Risdiplam is a medication used to treat spinal muscular atrophy (SMA), a genetic disorder characterized by weakness and wasting of the muscles needed for movement. It is designed for use in both children and adults. The drug works by increasing the production of the survival motor neuron (SMN) protein, which is crucial for muscle function.
How Risdiplam Works
Risdiplam is a small molecule that modifies splicing of the SMN2 gene to increase the production of functional SMN protein. This protein is essential for the maintenance and survival of motor neurons.
Administration and Dosage
Risdiplam is administered orally once a day after a meal, at the same time each day. The dosage varies based on age and weight:
For adults and children 2 years and older:
- Weight less than 20 kg: 0.25 mg/kg once daily
- Weight 20 kg or more: 5 mg once daily
For children under 2 years:
- Less than 2 months old: 0.15 mg/kg once daily
- 2 months to less than 2 years: 0.2 mg/kg once daily
Risdiplam is supplied as a powder that must be reconstituted into a liquid form at a pharmacy. It should not be used in its powder form. The mixed liquid medicine should be stored in the refrigerator and can be kept at room temperature for a limited period.
Potential Side Effects
Risdiplam can cause various side effects, some of which can be serious. Common side effects include:
- Fever
- Lung infection
- Vomiting
- Diarrhea
- Constipation
- Rash
- Cold symptoms (stuffy nose, sneezing, sore throat)
Serious side effects may include:
- Pain or burning when urinating
- Signs of a lung infection (fever, cough with mucus, chest pain, shortness of breath)
Precautions and Warnings
Risdiplam may harm an unborn baby, so effective birth control is necessary during treatment and for at least one month after the last dose. Women who are pregnant or planning to become pregnant should inform their healthcare provider. Additionally, risdiplam may affect fertility in men. Patients should not breastfeed while using risdiplam.
How to obtain the latest development progress of all drugs?
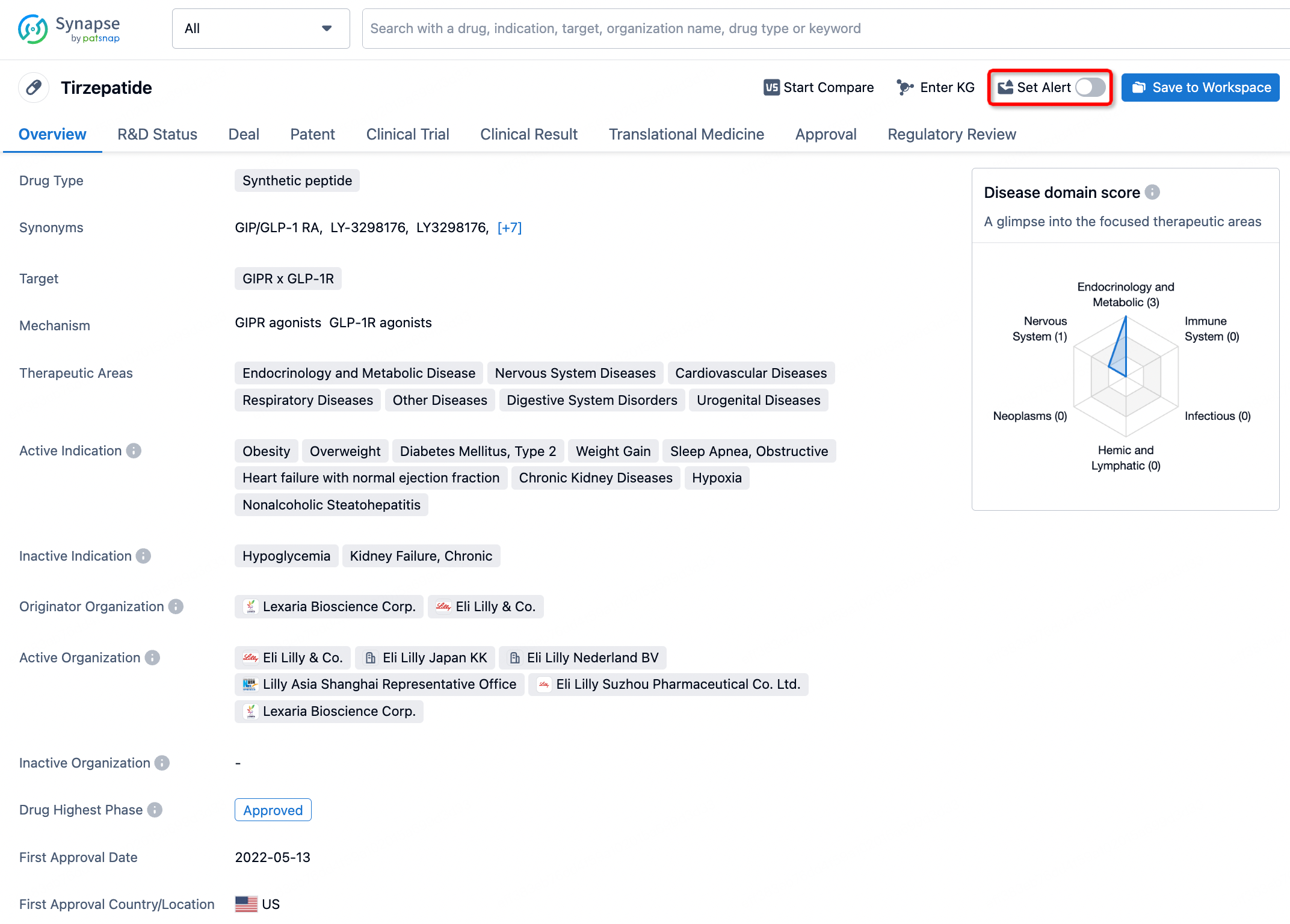
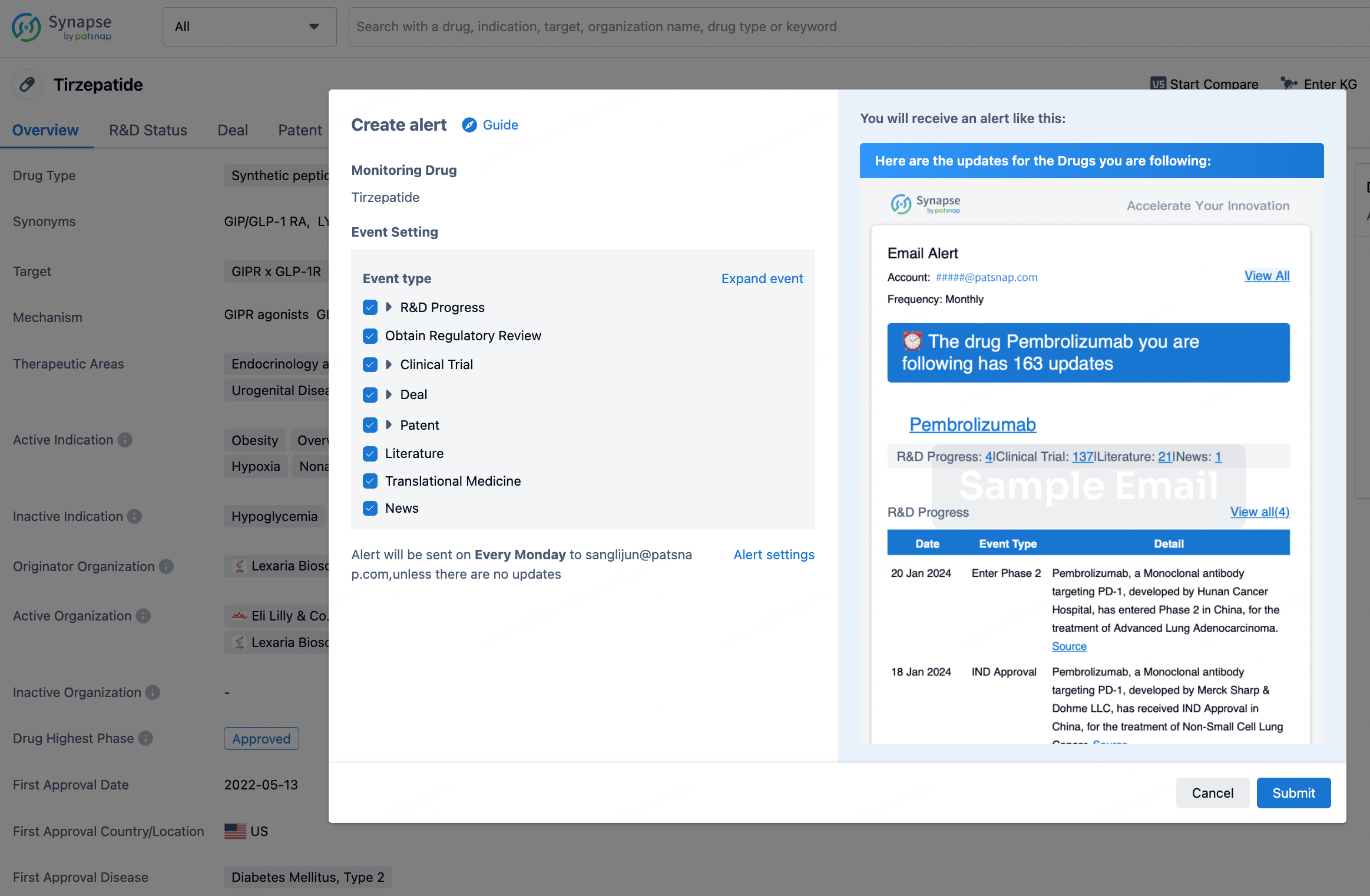
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!