Is Ritlecitinib approved by the FDA?
Ritlecitinib, marketed under the brand name Litfulo, received FDA approval on June 23, 2023, specifically for the treatment of severe alopecia areata. This approval marks an important development for patients suffering from this condition, providing a new therapeutic option to help manage hair loss.
Uses and Administration
- Indication: Ritlecitinib is used to treat severe alopecia areata, an autoimmune disorder that causes hair loss.
- Administration: The medication is taken once daily, with or without food. Patients should swallow the capsule whole and not crush, chew, break, or open it. Regular medical tests are required before and during treatment to monitor for infections and other potential side effects.
Side Effects
While ritlecitinib can be effective in treating alopecia areata, it may cause various side effects. Patients should be aware of both common and serious side effects and seek medical attention if necessary.
Common Side Effects:
- Headache
- Dizziness
- Fever
- Acne
- Skin rashes and itching
- Folliculitis (inflammation of hair follicles)
- Anemia
- Diarrhea
- Mouth ulcers and gum issues
Serious Side Effects:
- Signs of a heart attack: chest pain, pain spreading to the jaw or shoulder, nausea, sweating
- Signs of a blood clot: numbness or weakness on one side of the body, chest pain, vision or speech problems, pain or swelling in one leg, sudden cough or shortness of breath
- Signs of infection: fever, chills, sore throat, body aches, easy bruising or bleeding
- Signs of tuberculosis: cough, night sweats, loss of appetite, weight loss, fatigue
- Symptoms of herpes zoster: skin sores, itching, tingling, burning pain, rash on the face or torso
Patients experiencing any severe side effects should stop taking ritlecitinib and seek emergency medical help immediately.
Precautions and Warnings
- Infections: Ritlecitinib can increase the risk of serious or fatal infections. Patients should inform their doctor about any signs of infection or a history of tuberculosis. Blood tests for infections are required before starting treatment.
- Allergies: Patients allergic to ritlecitinib should not use this medication.
- Health Conditions: Inform your doctor if you have a history of lung disease, heart problems, liver disease, diabetes, cancer, or a weakened immune system. Smoking can also increase the risk of side effects.
- Vaccinations: Patients should be up to date on all vaccines before starting ritlecitinib and avoid live vaccines during treatment.
Drug Interactions
Ritlecitinib can interact with other medications, including prescription drugs, over-the-counter medicines, vitamins, and herbal products. Patients should inform their doctor about all other medicines they are using to avoid potential interactions.
Storage
Store ritlecitinib capsules at room temperature, away from moisture and heat. Keep the capsules in their original container with the moisture-absorbing preservative.
Conclusion
Ritlecitinib (Litfulo) offers a new treatment option for patients with severe alopecia areata, having received FDA approval on June 23, 2023. While effective, it is essential to be aware of the potential side effects and necessary precautions when using this medication. Always follow the prescribed instructions and consult with healthcare providers to ensure safe and effective use.
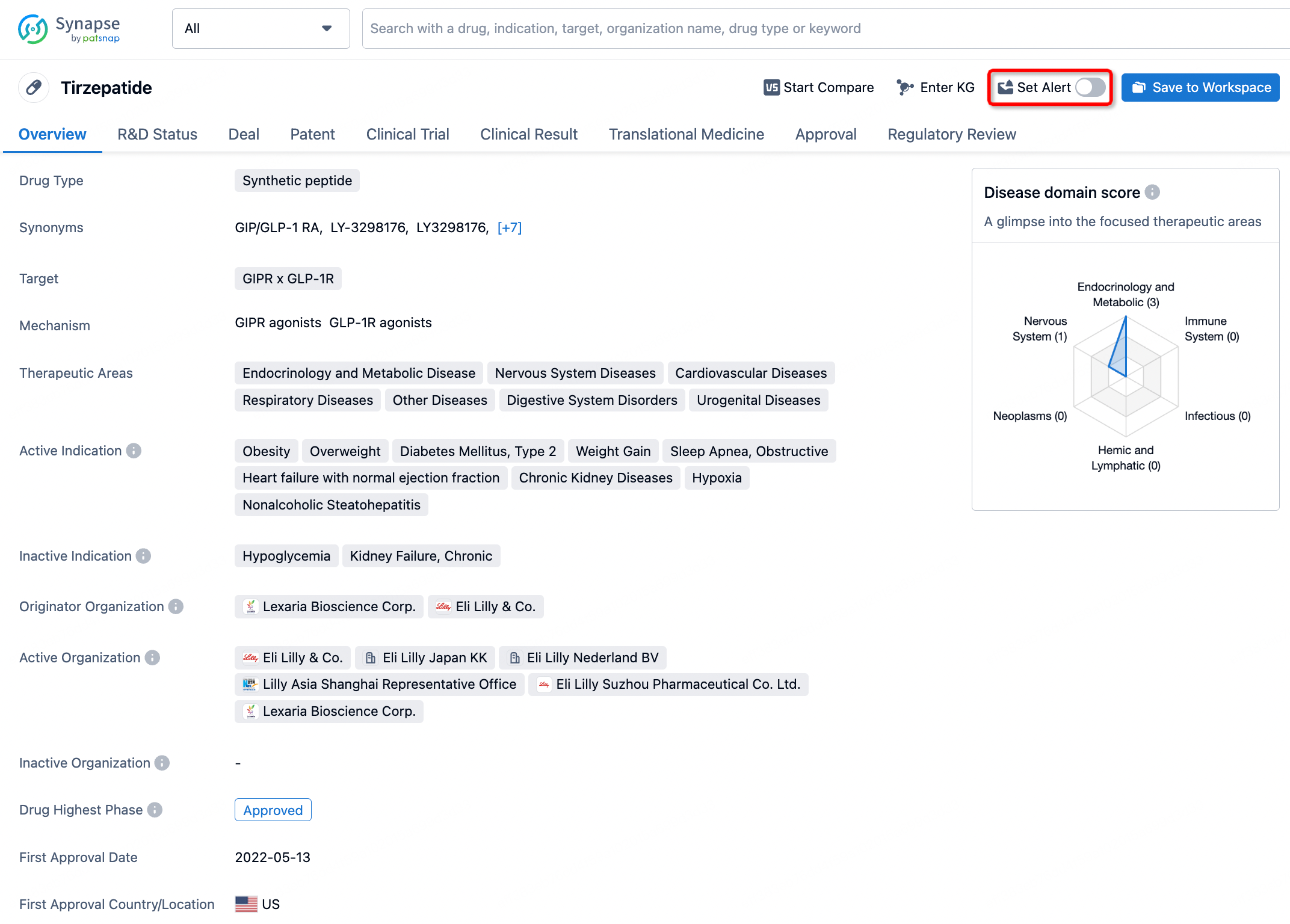
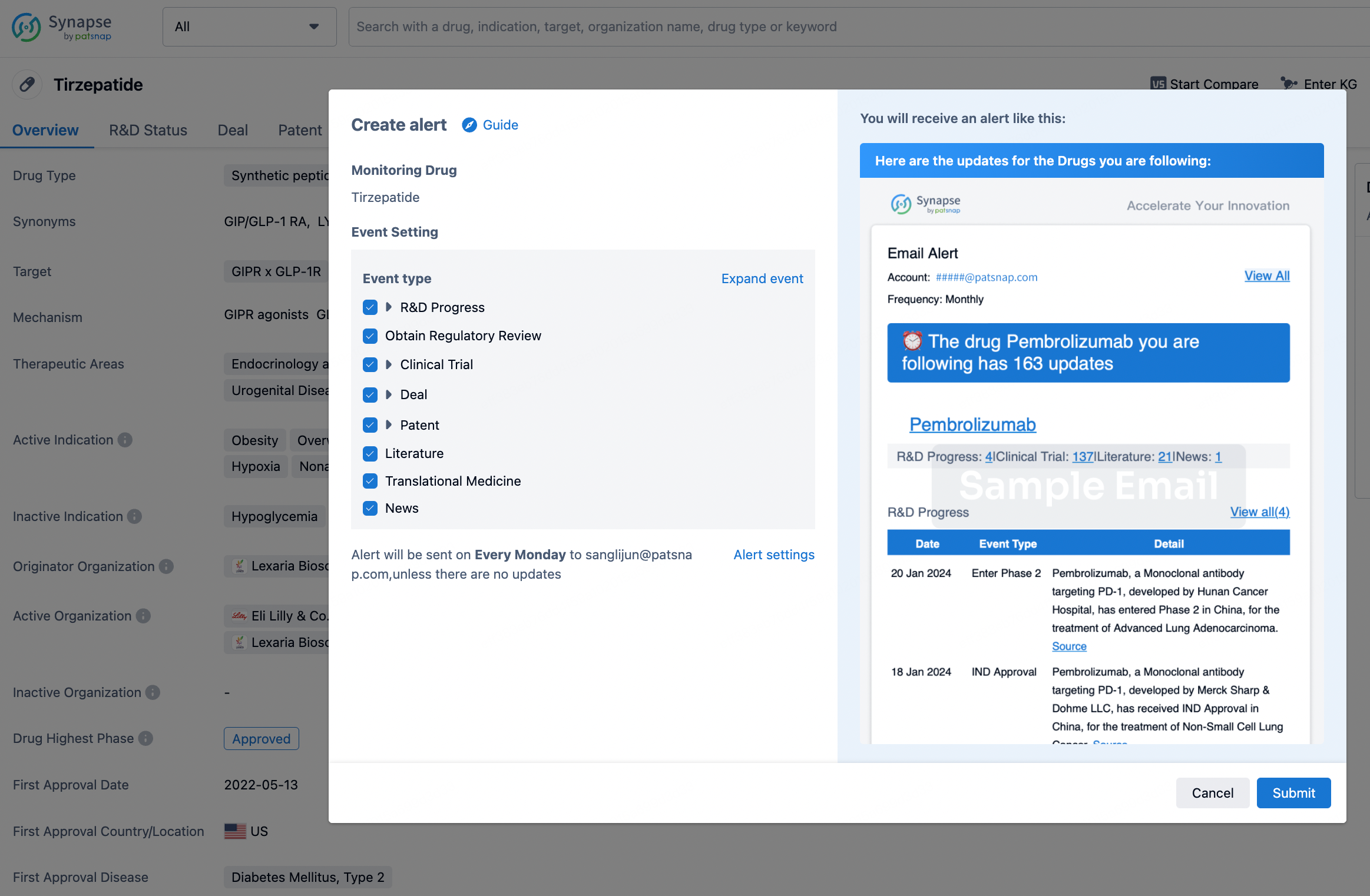
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!