Is Tenapanor approved by the FDA?
Yes, tenapanor is FDA approved. Tenapanor, marketed under the brand names Ibsrela and Xphozah, has been approved by the FDA for the treatment of irritable bowel syndrome with constipation (IBS-C) and for lowering phosphorus levels in the blood in adults with kidney disease who are on dialysis and have not responded to, or cannot take, phosphate binders.
What is Tenapanor?
Tenapanor belongs to the drug class of NHE3 inhibitors and is available in oral tablet form (20 mg, 30 mg, and 50 mg). It works by inhibiting the sodium/hydrogen exchanger 3 (NHE3) in the gut, which reduces sodium absorption and increases water secretion into the intestines, thereby softening stools and promoting bowel movements.
Indications for Use
- Irritable Bowel Syndrome with Constipation (IBS-C): Tenapanor is used in adults to treat IBS-C by alleviating symptoms such as abdominal pain and constipation.
- Hyperphosphatemia in Dialysis Patients: It is also prescribed to lower phosphorus levels in the blood in adults with kidney disease who are on dialysis.
Dosage and Administration
- For IBS-C: The usual adult dose is 50 mg taken orally twice a day, immediately before breakfast and dinner.
- For Hyperphosphatemia: The specific dosing and administration details for this condition should be followed as prescribed by a healthcare provider.
Side Effects
Common side effects of tenapanor may include:
- Gas
- Diarrhea
- Feeling of fullness or pressure in the abdomen
- Dizziness
Severe side effects include:
- Severe diarrhea
Patients are advised to seek emergency medical help if they experience signs of an allergic reaction, such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat.
Warnings and Precautions
- Contraindications: Do not use tenapanor if you have a bowel blockage.
- Age Restrictions: It should not be used in children younger than 6 years old.
- Pregnancy and Breastfeeding: Consult with a doctor if you are pregnant, planning to get pregnant, or breastfeeding.
Drug Interactions
Tenapanor can interact with various medications, including prescription and over-the-counter medicines, vitamins, and herbal products. It is crucial to inform your doctor about all the medications you are taking to avoid potential interactions.
Storage
Store tenapanor in its original container at room temperature, away from moisture and heat. Ensure the container is tightly closed and do not remove the moisture-absorbing preservative from the container.
Conclusion
Tenapanor is an FDA-approved medication for the treatment of IBS-C and for managing hyperphosphatemia in dialysis patients. Proper usage, adherence to prescribed dosages, and awareness of potential side effects and drug interactions are essential for the safe and effective use of tenapanor. For more detailed information and guidance, consult with a healthcare provider.
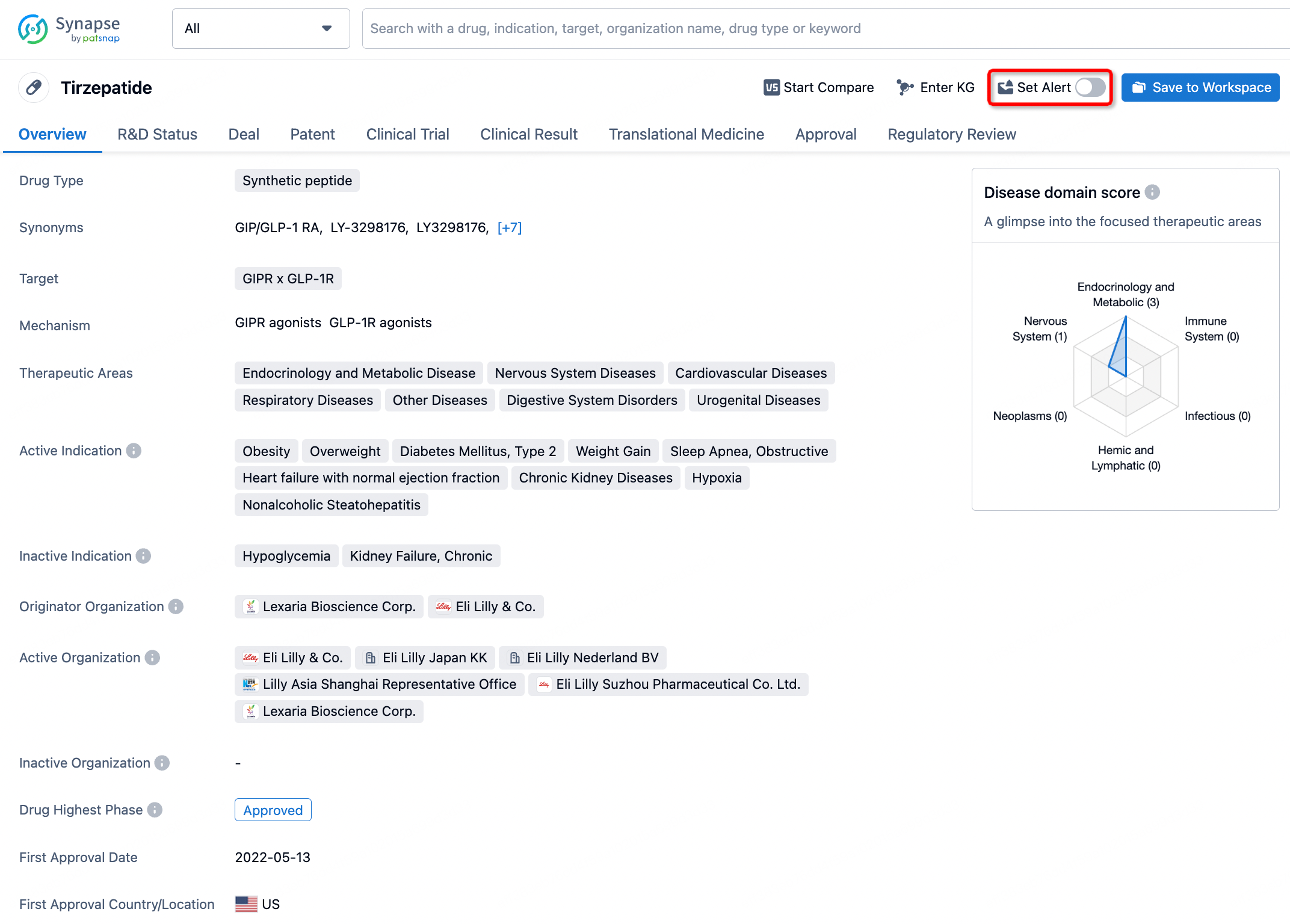
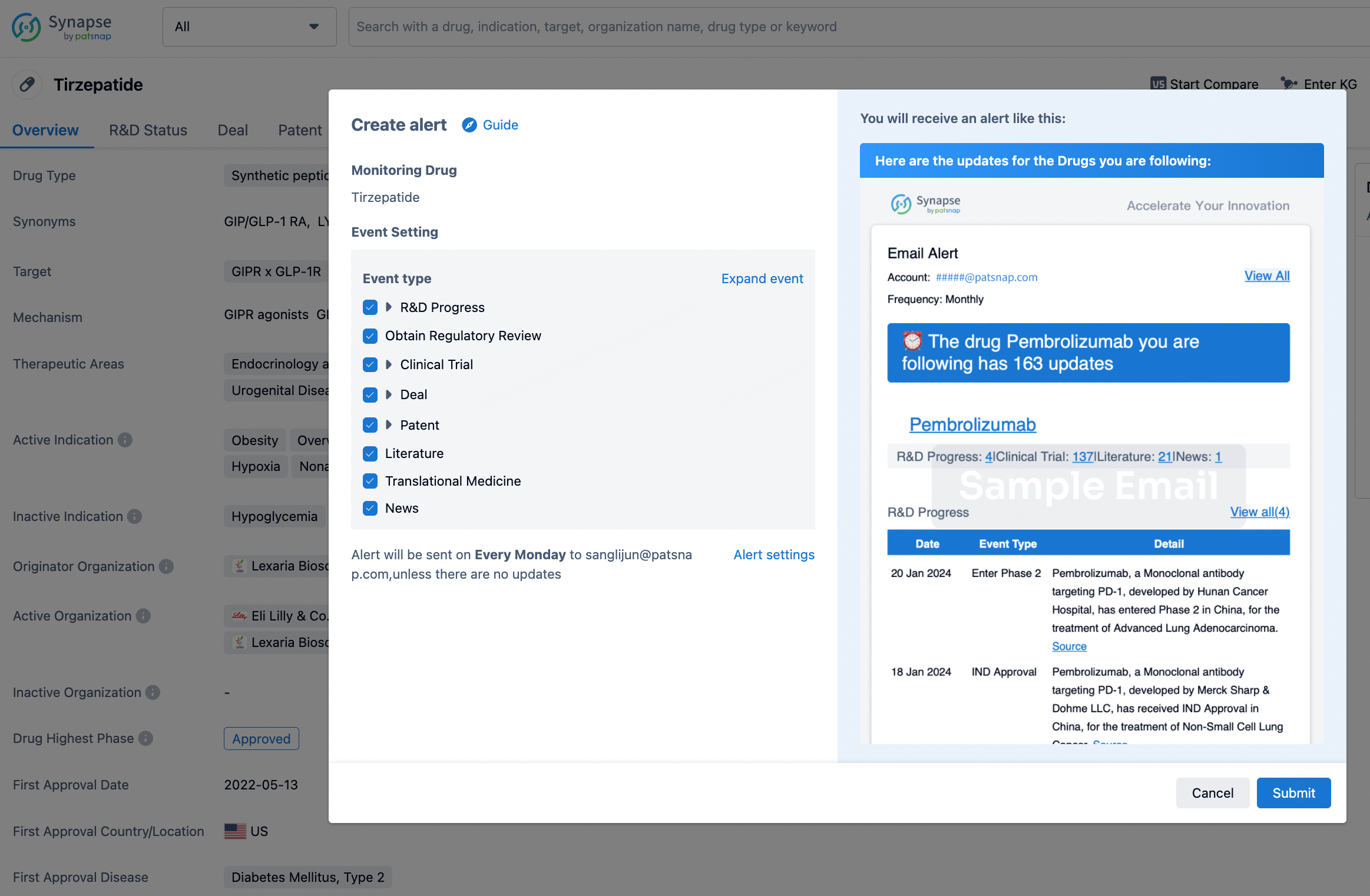
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!