Is Tofersen approved by the FDA?
Yes, Tofersen, marketed under the brand name Qalsody, received FDA approval on April 25, 2023, for the treatment of amyotrophic lateral sclerosis (ALS) in adults with a mutation in the superoxide dismutase 1 (SOD1) gene.
Administration
Tofersen is administered via intrathecal injection, meaning it is injected into the fluid surrounding the spinal cord. The administration process involves:
- Loading Dose: 100 mg intrathecally every 14 days for 3 doses.
- Maintenance Dose: 100 mg intrathecally every 28 days thereafter.
Side Effects
Common Side Effects
- Muscle or joint pain
- Muscle stiffness
- Fatigue
- Nerve or back pain
- Pain in hands or feet
- Increased white blood cells and protein in the fluid around the spine
Serious Side Effects
- Severe muscle pain and cramps
- Nerve pain (numbness, weakness, tingling, burning pain)
- Meningitis symptoms (fever, headache, neck stiffness, increased sensitivity to light, nausea, vomiting, confusion, drowsiness)
- Papilledema (blurry vision, swelling, vision changes)
- Increased intracranial pressure (severe headaches, ringing in the ears, dizziness, nausea, vision problems, pain behind the eyes)
Some side effects may occur during the injection, such as dizziness, nausea, light-headedness, itching, sweating, headache, chest tightness, back pain, trouble breathing, or swelling in the face.
Warnings and Precautions
- Allergies and Medical Conditions: Inform your doctor about any medical conditions or allergies before receiving tofersen.
- Pregnancy and Breastfeeding: The effects of tofersen on an unborn baby are unknown. Discuss with your doctor if you are pregnant or plan to become pregnant. Ask if it is safe to breastfeed while receiving tofersen.
- Drug Interactions: Inform your doctor about all medications, including prescription and over-the-counter medicines, vitamins, and herbal products, to avoid potential interactions.
Dosage Information
- Initial Dosing: 100 mg intrathecally every 14 days for the first three doses.
- Maintenance Dosing: 100 mg intrathecally every 28 days.
If a dose is missed, administer it as soon as possible and follow the subsequent schedule accordingly. Tofersen is administered by a healthcare provider, and frequent medical tests are required to monitor its effects.
Conclusion
Tofersen (Qalsody) was FDA approved on April 25, 2023, for the treatment of ALS in adults with an SOD1 gene mutation. It represents a significant advancement in the management of this specific form of ALS, providing a targeted therapeutic option for affected individuals.
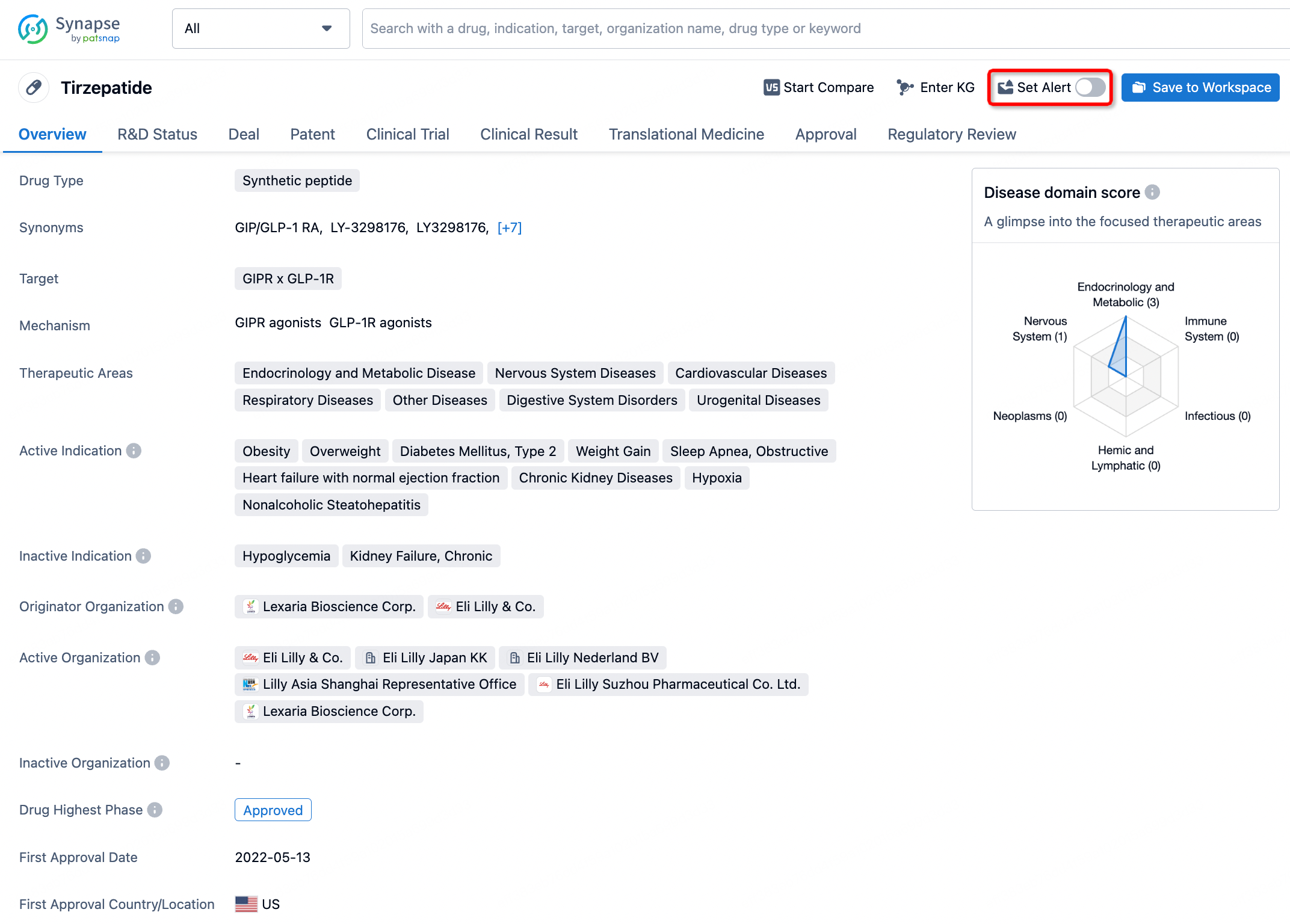
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!