Italfarmaco's Duvyzat™ Approved by FDA for Duchenne Muscular Dystrophy
Italfarmaco S.p.A. has revealed that Duvyzat™ (givinostat), an innovative inhibitor targeting histone deacetylase (HDAC), has received authorization by the United States Food and Drug Administration for use in treating individuals aged six and above diagnosed with Duchenne muscular dystrophy. This genetic disorder, linked to the X chromosome, causes progressive muscle degeneration and manifests with clinical signs beginning in early youth, significantly reducing life expectancy.
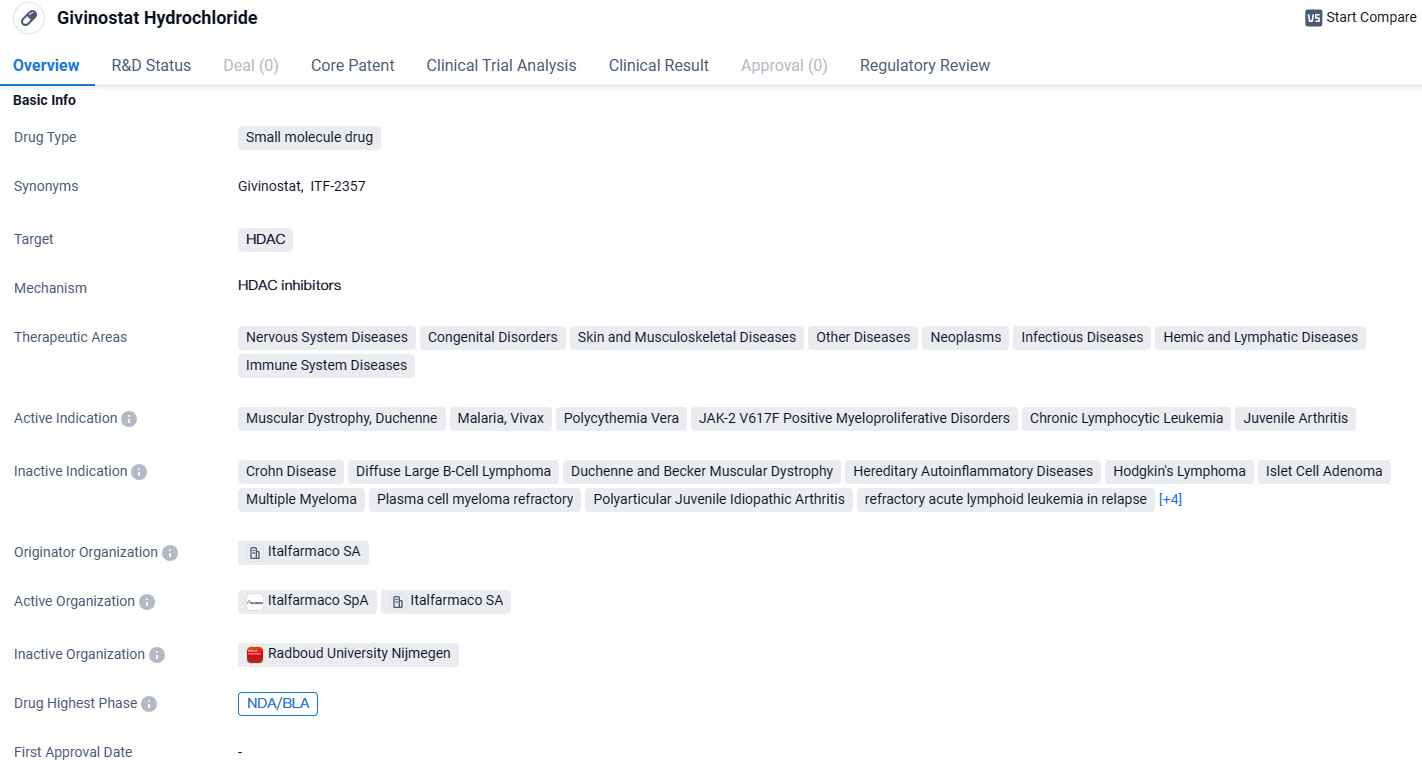
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
 "Italfarmaco Group's authorization of Duvyzat for the treatment of Duchenne Muscular Dystrophy (DMD) signifies our pledge to bring about a dependable and efficacious solution to those afflicted with DMD," expressed Paolo Bettica, MD, PhD, the company's Chief Medical Officer.
"Italfarmaco Group's authorization of Duvyzat for the treatment of Duchenne Muscular Dystrophy (DMD) signifies our pledge to bring about a dependable and efficacious solution to those afflicted with DMD," expressed Paolo Bettica, MD, PhD, the company's Chief Medical Officer.
"We owe a debt of gratitude to individuals affected by DMD and their unwavering care providers who were instrumental in achieving this significant FDA sanction. Our immediate goal is to ensure Duvyzat is accessible for those managing DMD across the United States without delay," confirmed Dr. Bettica.
Italfarmaco Group's leader Francesco De Santis, who is also the President of Italfarmaco Holding, remarked, "With the substantial therapeutic gap observed in Duchenne muscular dystrophy, the introduction of Duvyzat presents an opportunity to positively change the lives of a wide array of patients, regardless of the specific genetic mutation linked to their condition. This nod from the FDA underscores the relentless work and innovation of our scientific and clinical groups in reaching an essential turning point for our company."
The endorsement of Duvyzat stems from the data gleaned from the critical phase 3 EPIDYS study -a multicentre, randomized, double-blind trial against placebo, augmented by glucocorticosteroid therapy. With 179 young participants, aged six and above, the trial divided them between those receiving Duvyzat twice a day and those given a placebo. The success of EPIDYS was marked by a significant and substantial deviation in the time taken to perform the four-stair climb test in favor of the Duvyzat group.
In addition to the primary benefit, other secondary measures, such as the North Star Ambulatory Assessment and fat accumulation metrics via MRI, also showed promising results. Most side effects linked to Duvyzat were categorized as either mild or moderate. These findings were detailed in The Lancet Neurology in March 2024.
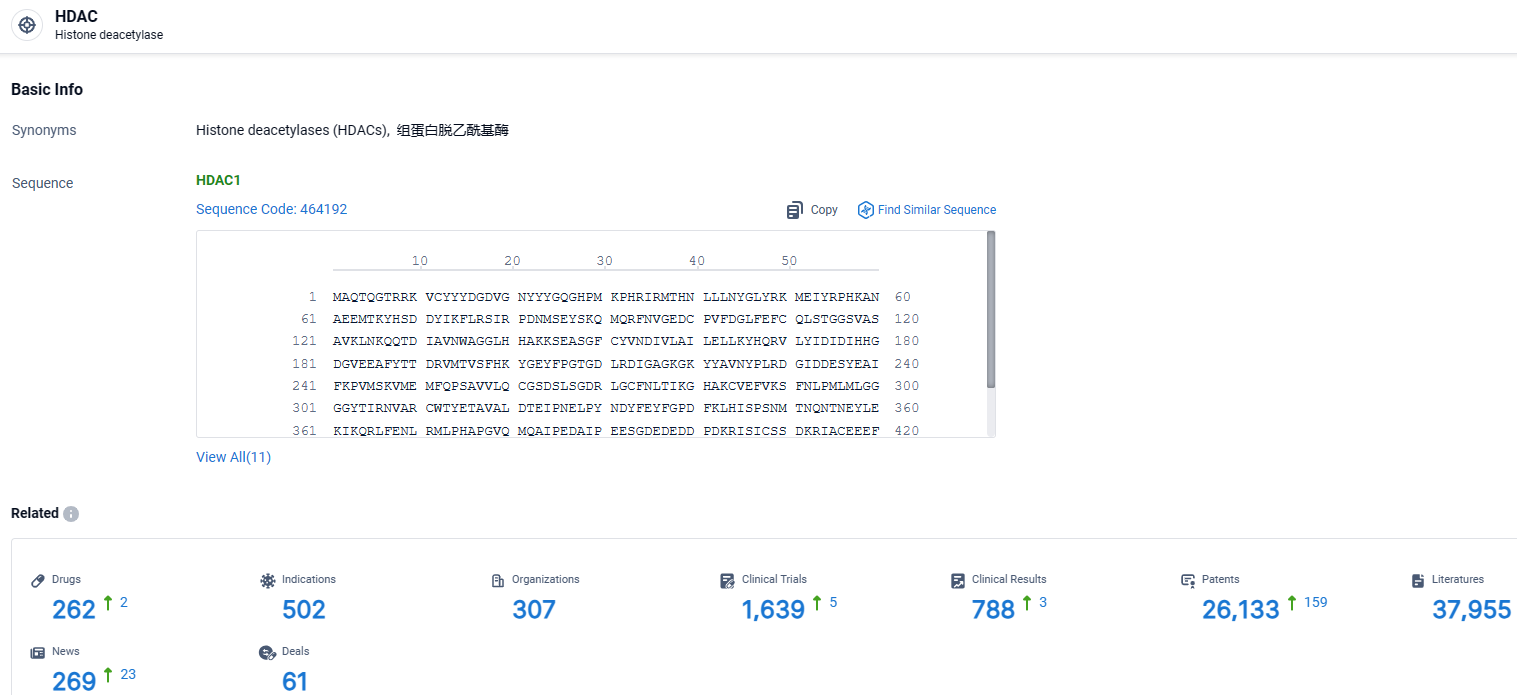
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 25 2024, there are 262 investigational drugs for the HDAC target, including 502 indications, 307 R&D institutions involved, with related clinical trials reaching 1639, and as many as 26133 patents.
Givinostat Hydrochloride is a small molecule drug that targets HDAC and has shown potential in a wide range of therapeutic areas. Its active indications cover various diseases, including muscular dystrophy, malaria, and different types of cancer. The drug has received regulatory designations that highlight its potential to address unmet medical needs. Further development and clinical trials will be necessary to determine the efficacy and safety of Givinostat Hydrochloride in treating these diseases.