Jazz Pharmaceuticals Releases Information on Stage 2 Research for Experimental JZP150 in Adults Suffering from PTSD
Jazz Pharmaceuticals plc has released preliminary findings from its Phase 2 study which assessed JZP150, a research-based selective inhibitor of the enzyme fatty acid amide hydrolase (FAAH), focusing on its effectiveness and safety profile for adults diagnosed with post-traumatic stress disorder.
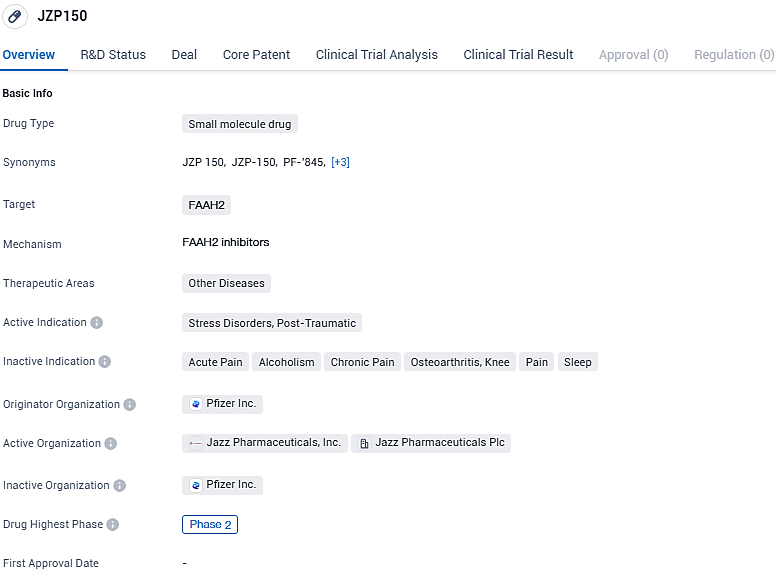
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The study failed to achieve its primary objective. A significant decrease in overall PTSD symptom intensity was not observed, as per the Clinician Administered PTSD Scale (CAPS), when comparing the effects of JZP150 with a placebo from the beginning of the trial to the 12th week. Furthermore, the study did not reach its important secondary objectives, which included the average alterations in Clinical Global Impression of Severity (CGI-S) and the Patient Global Impression of Severity scales from the starting point to the 12th week.
"We are sincerely thankful to everyone involved in making this study a reality, from the participating patients and their loved ones to our researchers and clinical team," expressed Rob Iannone, M.D., M.S.C.E., the executive vice president, and global head of research and development at Jazz Pharmaceuticals.
Rob Iannone added, "While we intend to perform a thorough examination of the collected data, the preliminary findings suggest that we are unlikely to proceed with further development of JZP150 for PTSD. We understand there's a crucial need for treatments in the PTSD community and are committed to communicating the results of this study with medical experts when appropriate."
The study's main goal was to track alterations in participants from the start to the 12th week utilizing the cumulative score from the CAPS-5.
This structured clinical dialogue is renowned as the benchmark for PTSD diagnosis and assessment. It is composed of 30 elements utilized by clinicians to determine the presence of PTSD and measure the severity of symptoms and their effect on the individual's day-to-day social and work performance. The study also monitored various secondary endpoints, such as score changes on the CGI-S and PGI-S from the start to the trial's conclusion.
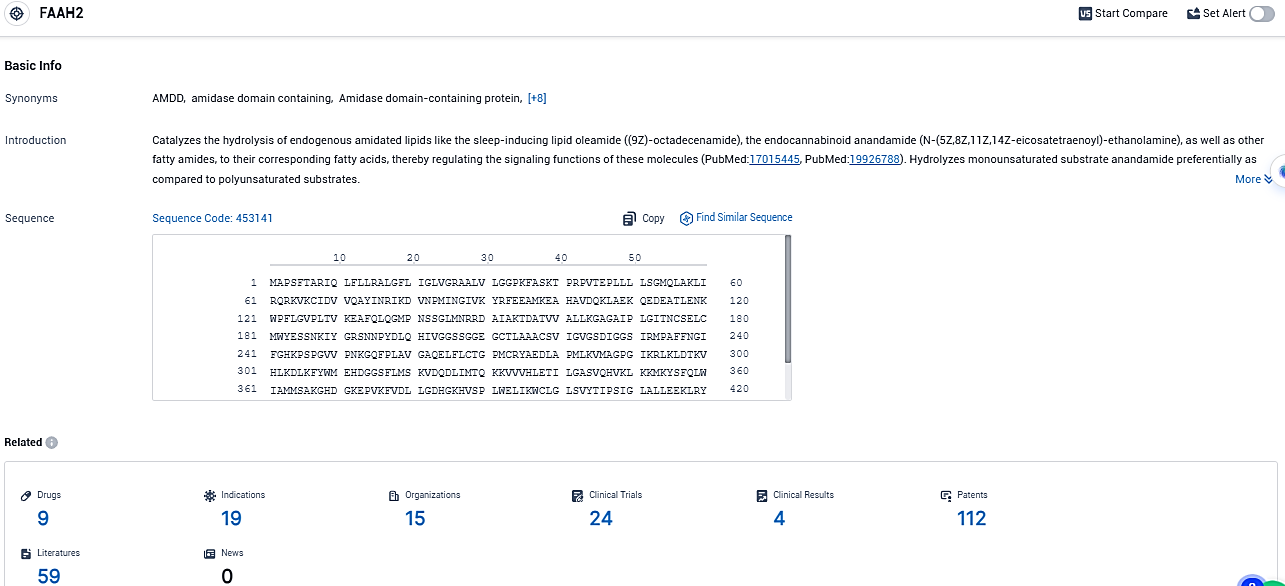
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 29, 2023, there are 9 investigational drugs for the FAAH2 target, including 19 indications, 15 R&D institutions involved, with related clinical trials reaching 24, and as many as 112 patents.
JZP150 targets FAAH2 and is being developed for the treatment of stress disorders, specifically PTSD. The drug is currently in Phase 2 of clinical development, indicating promising results in early testing. Further research and development are needed to determine the full potential of JZP150 in treating stress disorders and potentially other diseases.