WAINUA™ (eplontersen) Approved in US for Adult Polyneuropathy in Hereditary Transthyretin Amyloidosis
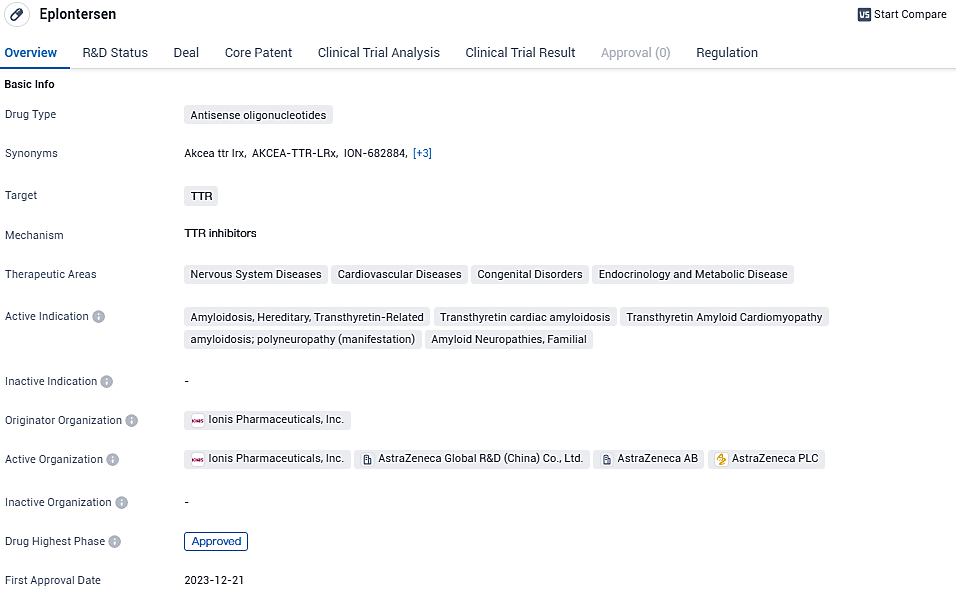
In the United States, the FDA has sanctioned the use of WAINUA™ (eplontersen), also known by its generic name eplontersen, which is a joint development by AstraZeneca and Ionis. This medication is specifically indicated for the therapeutic management of adults suffering from polyneuropathy due to hereditary transthyretin-mediated amyloidosis, often abbreviated as hATTR-PN or ATTRv-PN. WAINUA distinguishes itself as the sole therapeutic agent authorized for treating ATTRv-PN that patients can self-deliver using an auto-injector.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The sanction of WAINUA by the US FDA hinged on a favorable 35-week provisional review of data from the pivotal NEURO-TTRansform Phase III clinical study. In this examination, those who received WAINUA manifested a steady and marked improvement relating to the primary efficacy goals. These goals included the levels of transthyretin (TTR) protein in the serum and the extent of neuropathic detriment as scored by the revised Neuropathy Impairment Score +7. Furthermore, a principal secondary goal scrutinized the patients' life quality via the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy.
The esteemed Journal of the American Medical Association published the encouraging findings from the NEURO-TTRansform Phase III study, which highlighted WAINUA's efficacy in treating all stages of ATTRv-PN at different junctures—35, 66, and 85 weeks.
Michael J. Polydefkis, a notable Neurology Professor at Johns Hopkins University's medical division, commented: "Individuals dealing with hereditary TTR-related amyloid polyneuropathy often find their usual activities severely limited owing to the disease's persistent and deteriorating nature. The introduction of WAINUA into clinical practice is a significant step forward, offering renewed hope for patients afflicted by TTR-related amyloid polyneuropathy in maintaining their health."
ATTRv-PN is recognized for causing severe degradation of peripheral nerves that typically escalates into motor incapacity within a five-year post-diagnosis window and, if left untreated, often results in fatality after ten years. As a targeted therapeutic, WAINUA utilizes ligand-conjugated antisense oligonucleotide technology to attenuate TTR protein synthesis, thereby addressing both the inherited and wild type variants of TTR-related amyloidosis.
Eplontersen is under clinical investigation within the CARDIO-TTRansform Phase III trial as a prospective remedy for TTR-mediated amyloid cardiomyopathy—a relentless condition characterized by fatal, systemic amyloid protein buildup that often culminates in heart failure and mortality within three to five years after disease emergence.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
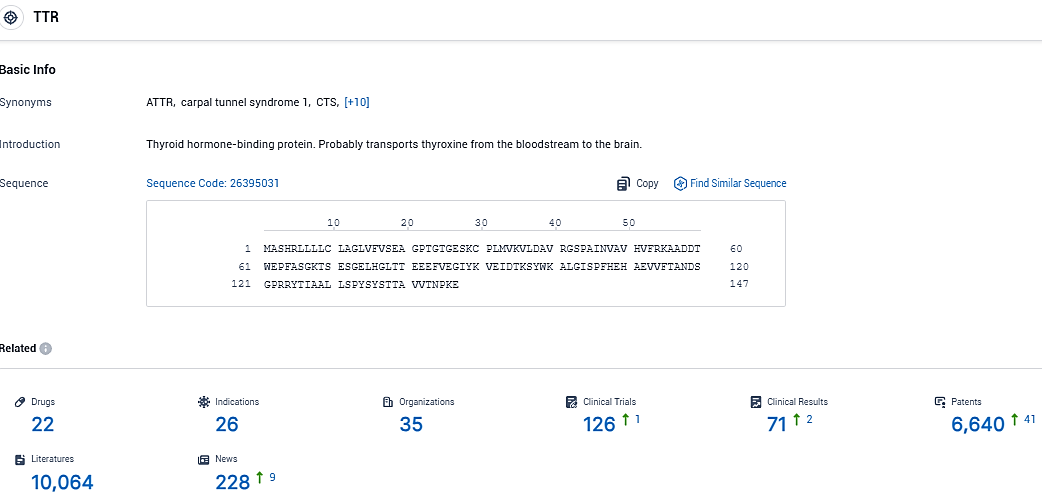
According to the data provided by the Synapse Database, As of December 29, 2023, there are 22 investigational drugs for the TTR target, including 26 indications, 35 R&D institutions involved, with related clinical trials reaching 126, and as many as 6640 patents.
Eplontersen targets the TTR protein and is indicated for the treatment of various diseases in the nervous system, cardiovascular system, and other areas. The drug has reached the approved phase globally and is currently in Phase 3 in China. Its first approval is expected in December 2023 in the United States. Eplontersen is regulated as an orphan drug, and Ionis Pharmaceuticals, Inc. is the originator organization behind its development.