Johnson & Johnson Seeks FDA Approval for TREMFYA® in Treating Severe Crohn’s Disease
Johnson & Johnson announced the submission of a supplemental Biologics License Application to the U.S. Food and Drug Administration seeking approval of TREMFYA (guselkumab) for the treatment of adults with moderately to severely active Crohn’s disease. This marks the second submission to the FDA for TREMFYA in inflammatory bowel disease this year following an application in March for moderately to severely active ulcerative colitis.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The latest submission includes results from the Phase 3 GALAXI program,1 which was featured as a late-breaking oral presentation at Digestive Disease Week 2024 last month.2 The GALAXI 2 and GALAXI 3 studies were the first-ever double-blind registrational head-to-head trials to demonstrate superiority versus ustekinumab in Crohn’s disease.
TREMFYA successfully met the co-primary endpoints for both SC maintenance doses compared to placebo in each individual study and demonstrated superiority to ustekinumab in multiplicity-controlled endoscopic endpoints based on data pooled from both studies.
The submission also includes results from the Phase 3 GRAVITI investigational study of TREMFYA SC induction therapy in adult patients with moderately to severely active Crohn’s disease, which met the co-primary endpoints, achieving statistically significant and clinically meaningful outcomes for clinical remission at Week 12 as well as endoscopic response at Week 12.
In addition, all multiplicity-controlled endpoints were met compared to placebo at Week 12, Week 24 and Week 48.4 The results from GALAXI and GRAVITI show that TREMFYA has the potential to become the only IL-23 inhibitor to offer both subcutaneous or intravenous induction options for the treatment of Crohn’s disease, and, if approved, will offer choice and versatility for patients and providers.
“Building upon nearly three decades of leadership and innovation in immunology, we are committed to addressing the needs of people living with Crohn’s disease through deep, scientific expertise and through our continued pioneering advances in the IL-23 pathway,” said David Lee, M.D., Ph.D., Global Therapeutic Area Head Immunology, Johnson & Johnson Innovative Medicine.
TREMFYA is the first approved fully-human, dual-acting monoclonal antibody that blocks IL-23 while also binding to CD64, a receptor on cells that produce IL-23. IL-23 is a cytokine secreted by activated monocyte/macrophages and dendritic cells that is known to be a driver of immune-mediated diseases including Crohn’s disease.
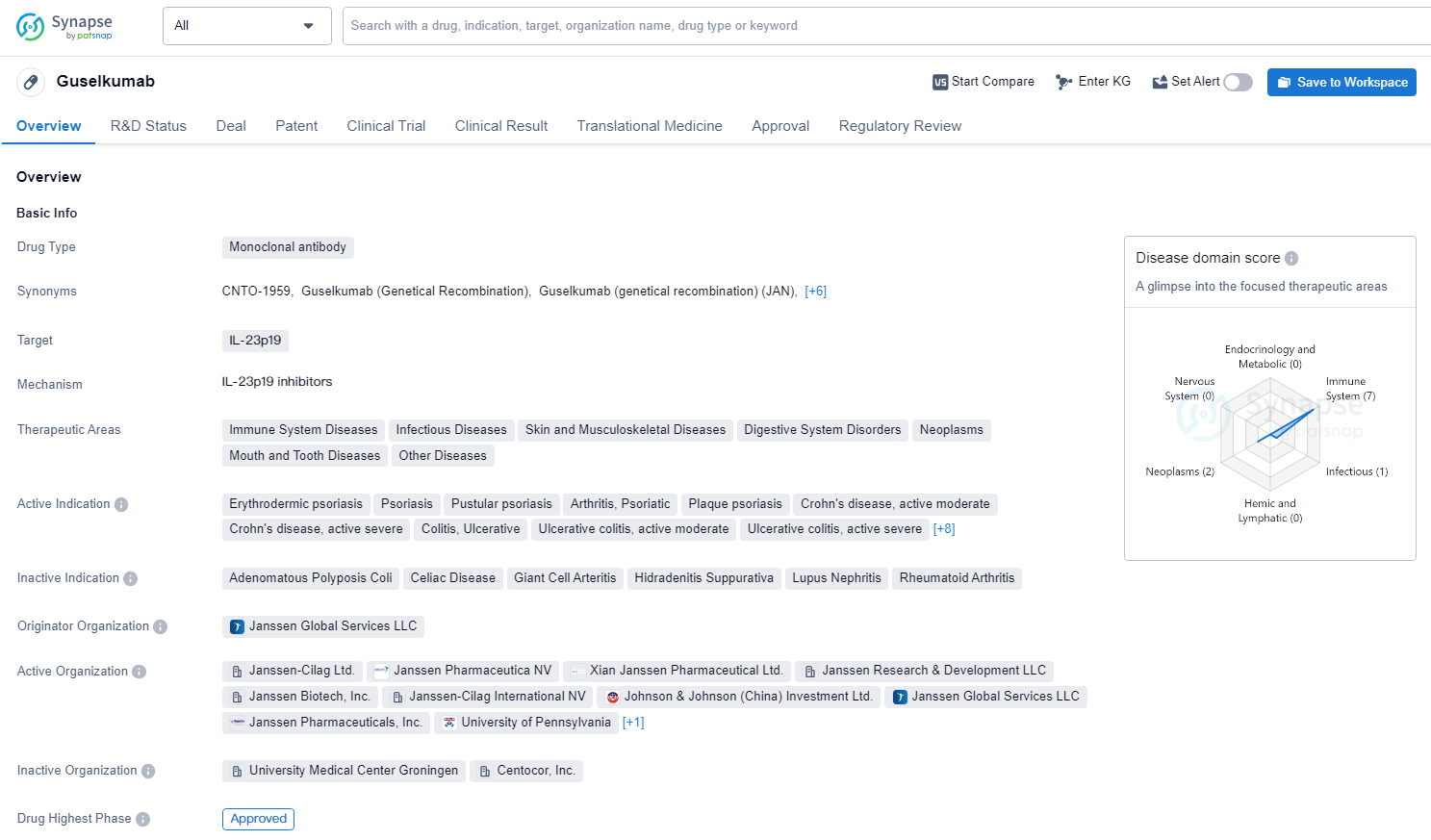
TREMFYA, the first-in-class IL-23 inhibitor, received U.S. FDA approval in July 2017 for the treatment of adult patients with moderate-to-severe plaque psoriasis and was subsequently approved for adults with active psoriatic arthritis in July 2020.
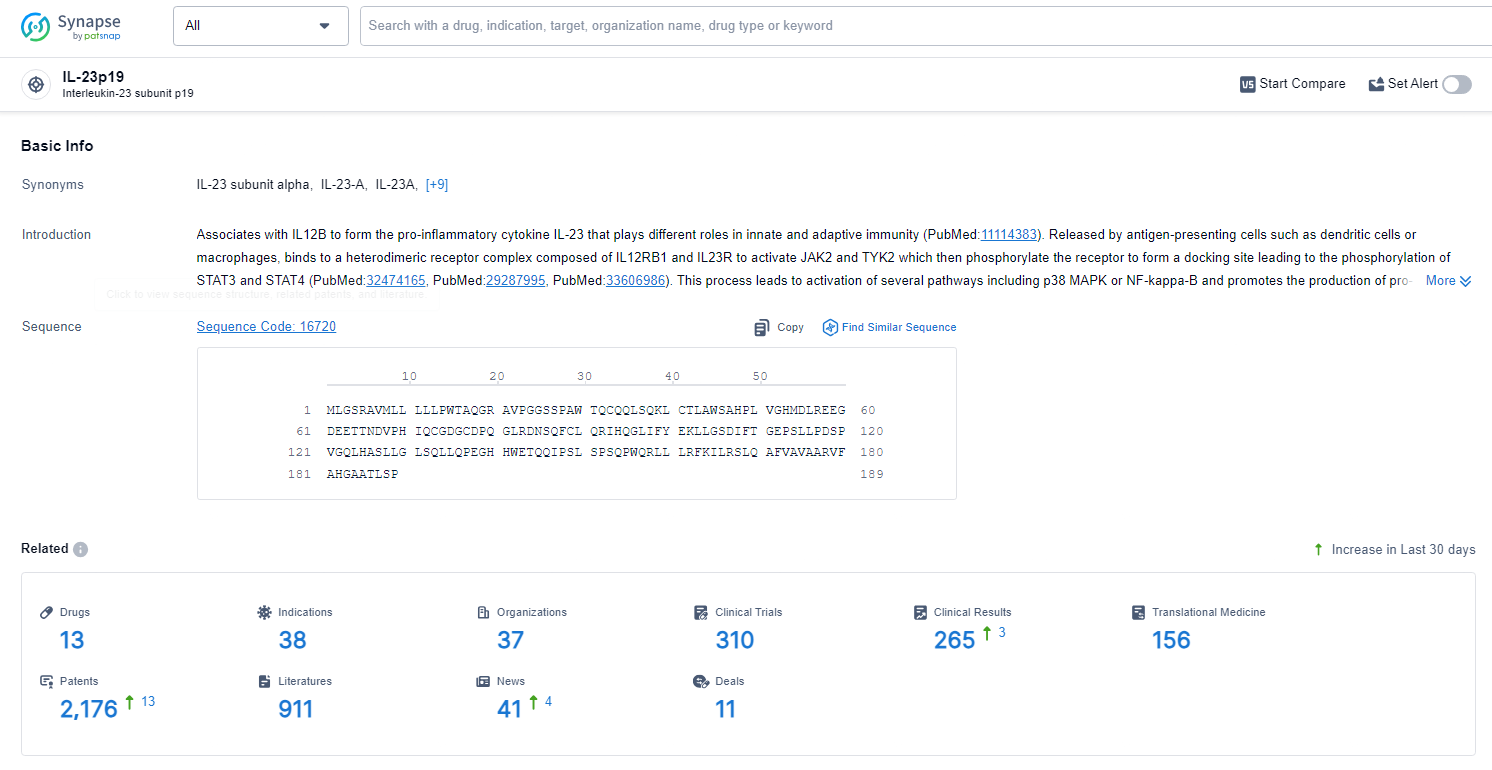
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 24, 2024, there are 13 investigational drugs for the IL-23p19 target, including 39 indications, 39 R&D institutions involved, with related clinical trials reaching 3, and as many as 2176 patents.
Guselkumab is a monoclonal antibody drug that targets IL-23p19 and is used in the treatment of a wide range of therapeutic areas, including immune system diseases, infectious diseases, skin and musculoskeletal diseases, digestive system disorders, neoplasms, mouth and tooth diseases, other diseases, nervous system diseases, and cardiovascu lar diseases. Guselkumab represents a significant advancement in the field of biomedicine, particularly in the treatment of immune-mediated and inflammatory diseases. Its approval and regulatory designations position it as a valuable addition to the pharmaceutical industry's efforts to address complex and challenging medical conditions.