Johnson & Johnson Seeks Initial Approval for Nipocalimab in Treating Broad-Spectrum Myasthenia Gravis
Johnson & Johnson (NYSE: JNJ) has reported the filing of a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA). This application aims to obtain the world's first approval for nipocalimab for the treatment of individuals affected by generalized myasthenia gravis (gMG).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The application incorporated data from the Phase 3 Vivacity-MG3 trial, which revealed that the results for a diverse group of antibody-positive participants receiving nipocalimab along with standard of care (SOC) surpassed those receiving a placebo plus SOC. The primary endpoint of the study assessed the improvement in the MG-ADL score from baseline over a 24-week period. The study included adults positive for anti-AChR, anti-MuSK, and anti-LRP4 antibodies, which represent about 95% of the generalized myasthenia gravis (gMG) patient population. This makes Vivacity-MG3 the first study to exhibit continuous disease control in these subtypes. Safety and tolerability were aligned with other nipocalimab studies.
“We are optimistic about nipocalimab's potential to provide durable disease control for individuals with generalized myasthenia gravis, a chronic, lifelong condition,” stated Bill Martin, Ph.D., Global Therapeutic Area Head, Neuroscience, Johnson & Johnson Innovative Medicine. “Filing for nipocalimab's approval marks a critical advancement as Johnson & Johnson persistently pushes the frontiers of research to develop pioneering treatments for autoantibody-driven diseases, leveraging decades of expertise in neuroscience and immunology. We anticipate constructive collaboration with the FDA in their evaluation of the supporting data.”
Nipocalimab remains the first and only FcRn blocker to show sustained disease control as measured by MG-ADL improvements when added to SOC compared to placebo plus SOC over six months of consistent dosing (every other week). This represents the longest period of controlled safety and efficacy evaluation of an FcRn blocker in gMG.
Earlier this year, Johnson & Johnson presented data at the American Academy of Neurology Annual Meeting emphasizing the molecular characteristics of nipocalimab. Its high binding affinity to the IgG binding site of FcRn may distinguish it within the FcRn blocker class of treatments. These properties, along with the dosing regimen selected for the study, are proposed to reduce IgG, including IgG autoantibodies in conditions such as gMG and other autoantibody-driven diseases.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
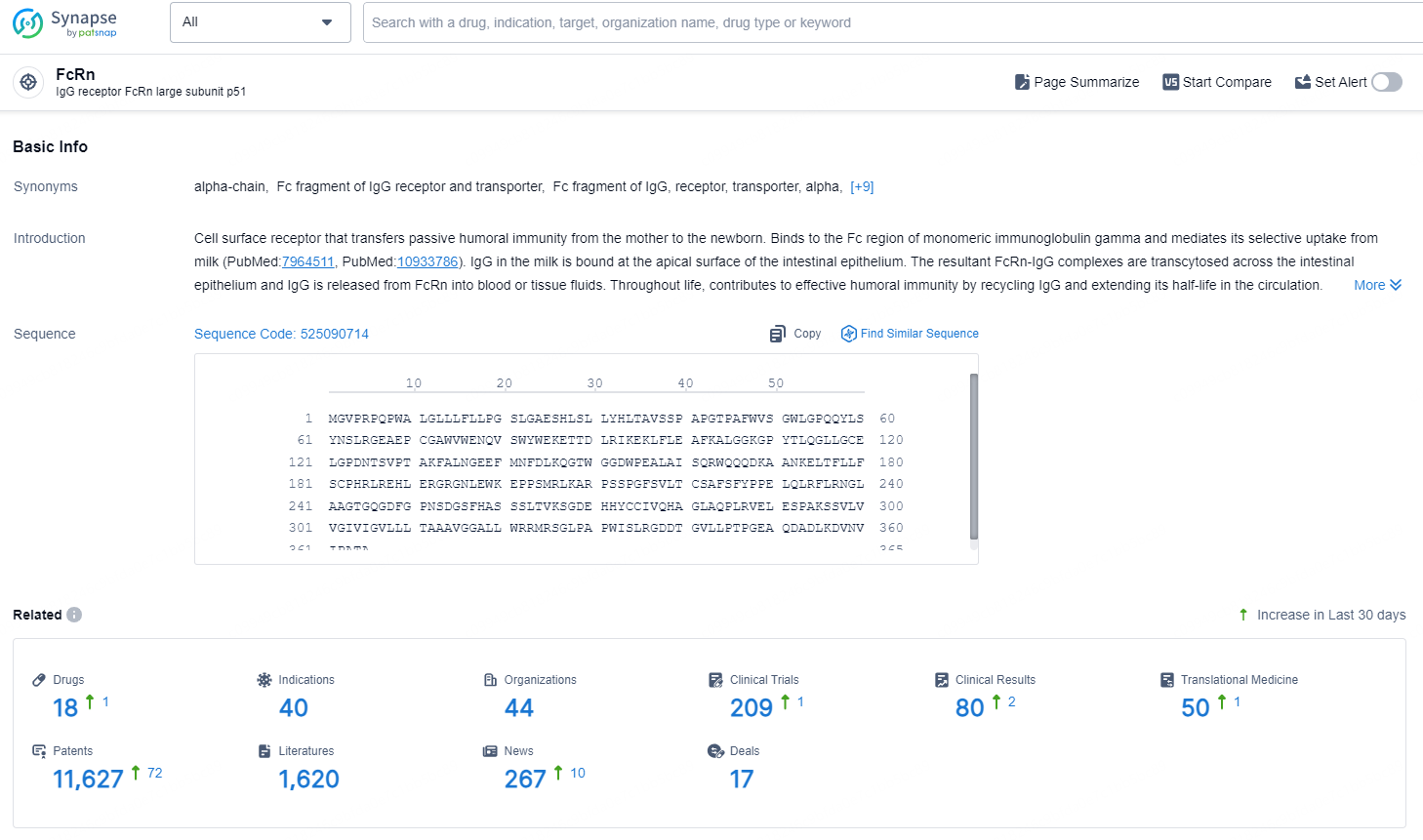
According to the data provided by the Synapse Database, As of September 3, 2024, there are 18 investigational drugs for the FcRn target, including 40 indications, 44 R&D institutions involved, with related clinical trials reaching 209, and as many as 11627 patents.
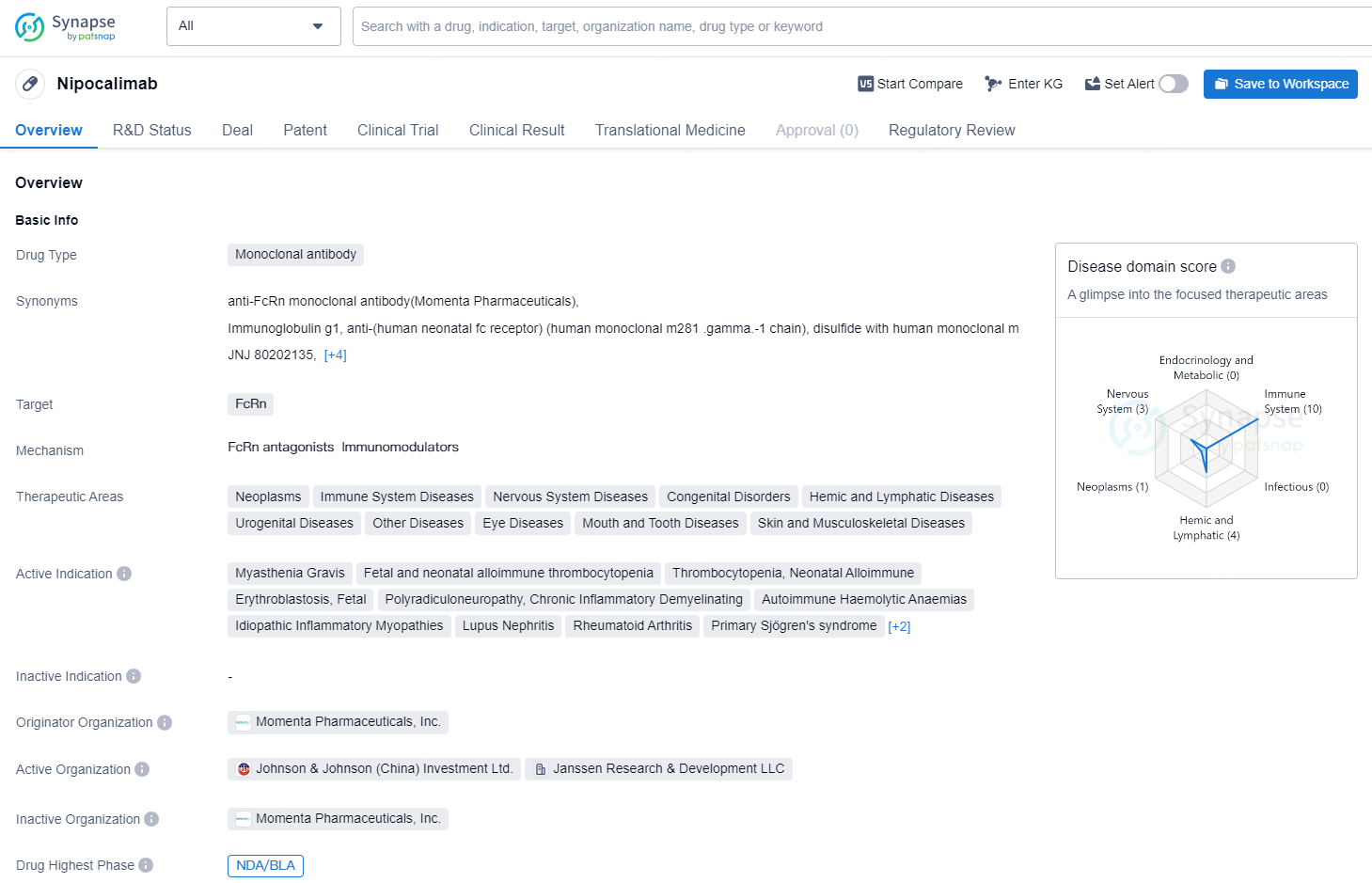
Nipocalimab is a monoclonal antibody drug that targets FcRn and is being developed by Momenta Pharmaceuticals, Inc. The drug has a wide range of therapeutic areas, including neoplasms, immune system diseases, nervous system diseases, congenital disorders, hemic and lymphatic diseases, urogenital diseases, eye diseases, mouth and tooth diseases, and skin and musculoskeletal diseases.