Merck Releases Progress Report for Phase 3 Studies: KEYNOTE-867 & KEYNOTE-630 Clinical Trials
Merck Corporation (ticker symbol: MRK on the NYSE), commonly referred to as MSD in territories excluding the United States and Canada, has recently divulged advancements pertaining to two pivotal Phase 3 clinical trials, designated as KEYNOTE-867 and KEYNOTE-630. Notably, Merck has announced the termination of the Phase 3 KEYNOTE-867 study, which was evaluating the efficacy of KEYTRUDA® (pembrolizumab), its anti-PD-1 therapeutic agent, in conjunction with stereotactic body radiotherapy (SBRT) for the management of patients diagnosed with early-stage non-small cell lung cancer (NSCLC) of stage I or II (specifically, stage IIB N0, M0), encompassing those who are medically unfit for surgery or have declined surgical intervention.
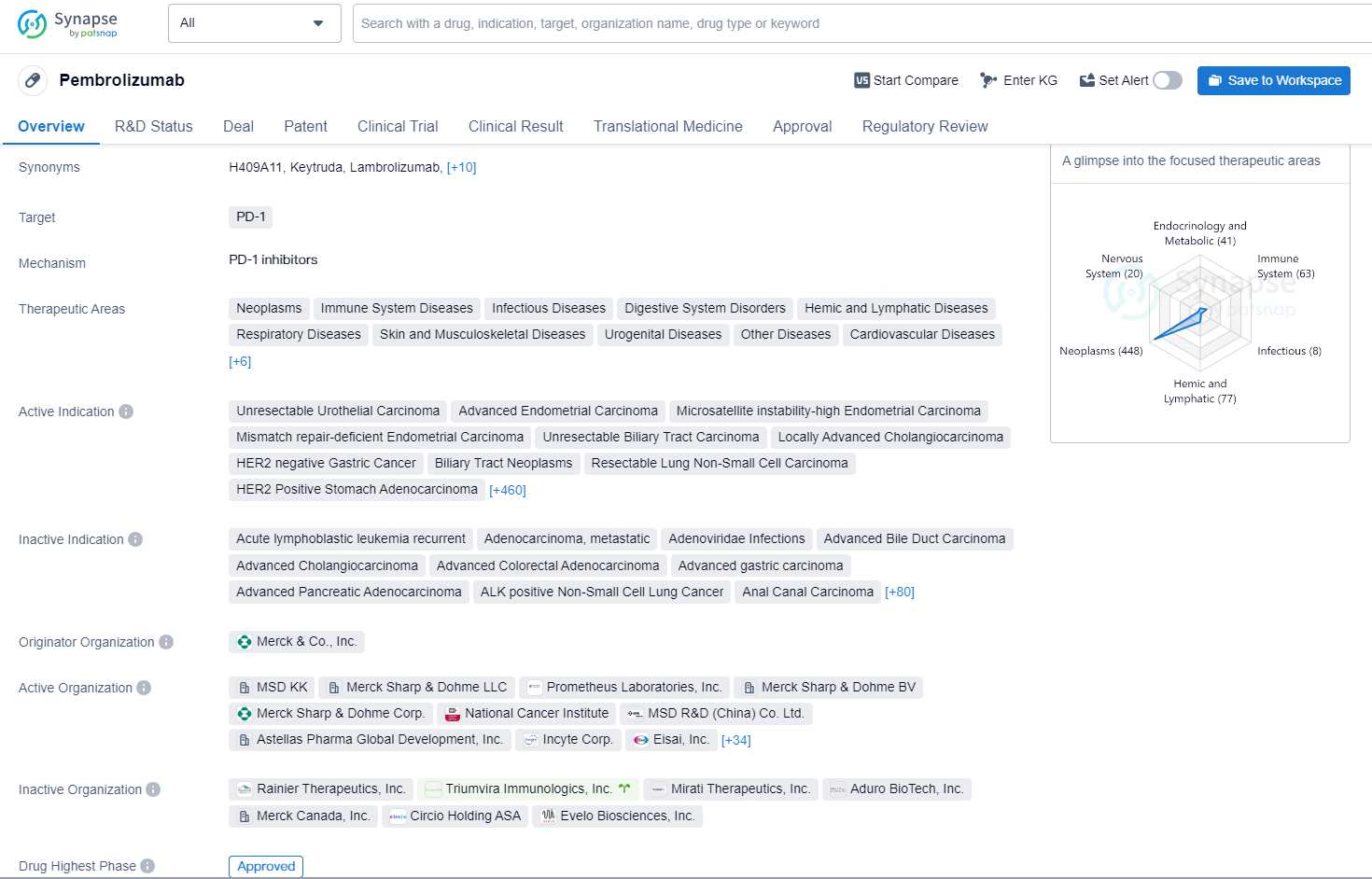
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This determination stems from the advisory of an impartial Data Monitoring Committee (DMC), having conducted a thorough assessment of the interim data as per the predefined plan. The interim evaluation revealed that the combination of KEYTRUDA and SBRT failed to exhibit a favorable impact on both event-free survival (EFS) and overall survival (OS), the primary and key secondary endpoints respectively, when juxtaposed against placebo plus SBRT. Furthermore, the risk-benefit assessment of this combination did not justify the continuation of the trial. Notably, the combination therapy was linked to a heightened incidence of adverse events (AEs), including fatal AEs, in comparison to SBRT and placebo.
Similarly, Merck has opted to halt the Phase 3 KEYNOTE-630 trial, investigating KEYTRUDA as an adjuvant therapy for high-risk, locally advanced cutaneous squamous cell carcinoma (cSCC) patients post-surgery and radiation, adhering to the guidance of an independent DMC. The DMC advised cessation due to a lack of promising outcomes, as the risk-benefit ratio did not support further progression. Preliminary analysis data indicated that KEYTRUDA did not achieve statistical significance in recurrence-free survival (RFS), the primary endpoint. While overall survival (OS), the key secondary endpoint, was not formally evaluated, the available results did not favor KEYTRUDA over placebo. The safety profile of KEYTRUDA in this trial remained consistent with its established safety record.
Merck has communicated this decision to the study investigators and encourages patients enrolled in these trials to consult with their study team and physicians regarding subsequent steps and treatment alternatives. The data analysis for both KEYNOTE-867 and KEYNOTE-630 is still underway, and the findings will be disseminated to the scientific fraternity and regulatory bodies.
"The landscape of cancer treatment has undergone rapid advancements in recent times, yet there persist unmet needs across diverse cancer types and stages," remarked Dr. Marjorie Green, Senior Vice President and Head of Oncology, Global Clinical Development, Merck Research Laboratories. "This underscores our relentless pursuit of innovative therapeutic strategies for cancers with high unmet needs, such as non-small cell lung cancer and cutaneous squamous cell carcinoma, aiming to benefit an even broader patient population. We express our profound gratitude to all patients, caregivers, and investigators for their invaluable contributions to these studies."
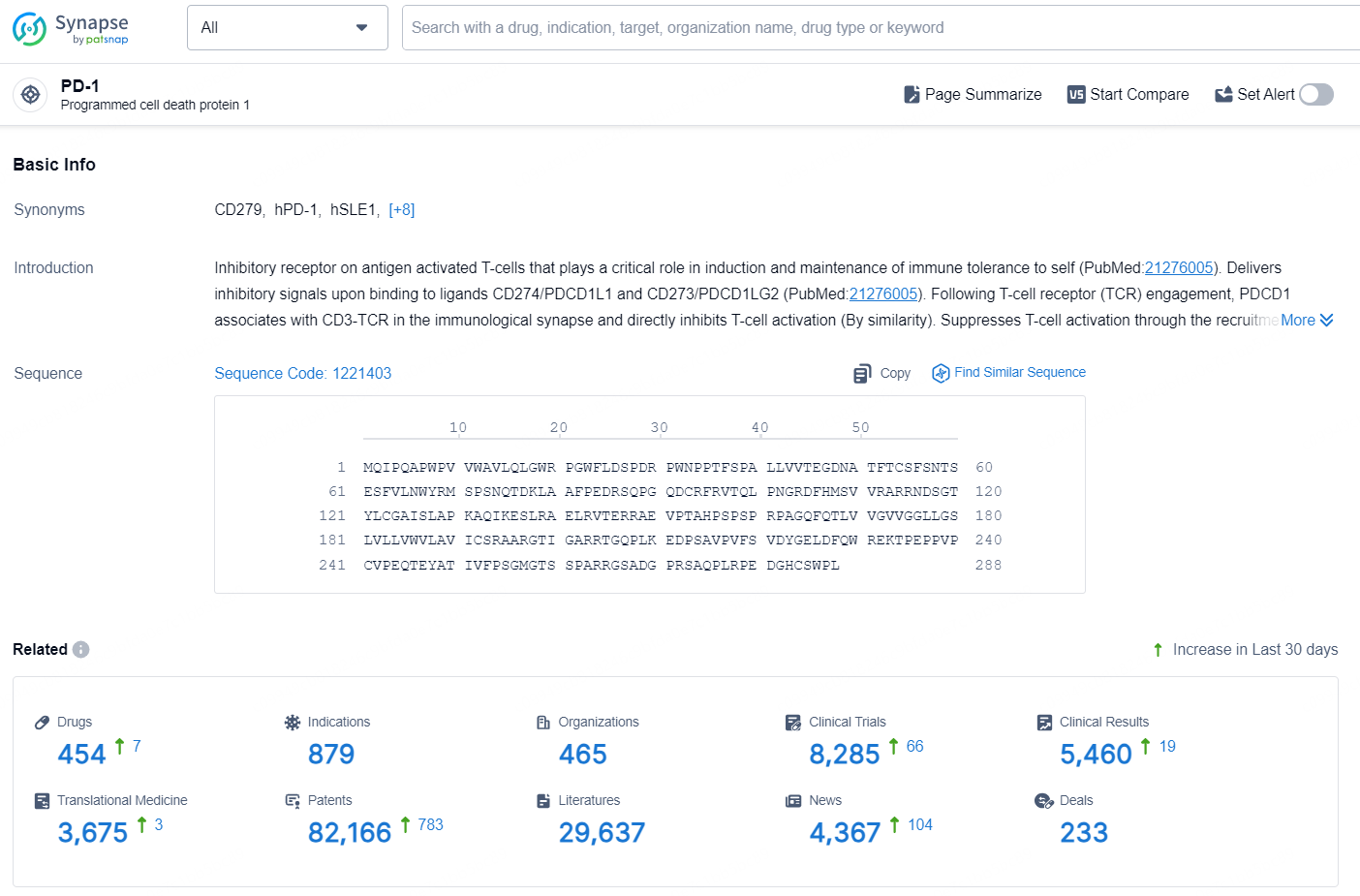
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 2, 2024, there are 119 investigational drugs for the PD-1 targets, including 62 indications, 131 R&D institutions involved, with related clinical trials reaching 567, and as many as 10743 patents.
KEYTRUDA is an anti-programmed death receptor-1 (PD-1) therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. KEYTRUDA is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD- L1 and PD-L2, thereby activating T lymphocytes which may affect both tumor cells and healthy cells.
Merck has the industry’s largest immuno-oncology clinical research program. There are currently more than 1,600 trials studying KEYTRUDA across a wide variety of cancers and treatment settings. The KEYTRUDA clinical program seeks to understand the role of KEYTRUDA across cancers and the factors that may predict a patient’s likelihood of benefitting from treatment with KEYTRUDA, including exploring several different biomarkers.