Johnson & Johnson to Purchase Proteologix, Inc. to Lead in Eczema Treatment
Johnson & Johnson revealed it has executed a definitive agreement to purchase Proteologix, Inc., a privately-owned biotechnology firm specializing in bispecific antibodies aimed at immune-mediated diseases, for $850 million in cash. There is also the potential for an additional milestone payment.
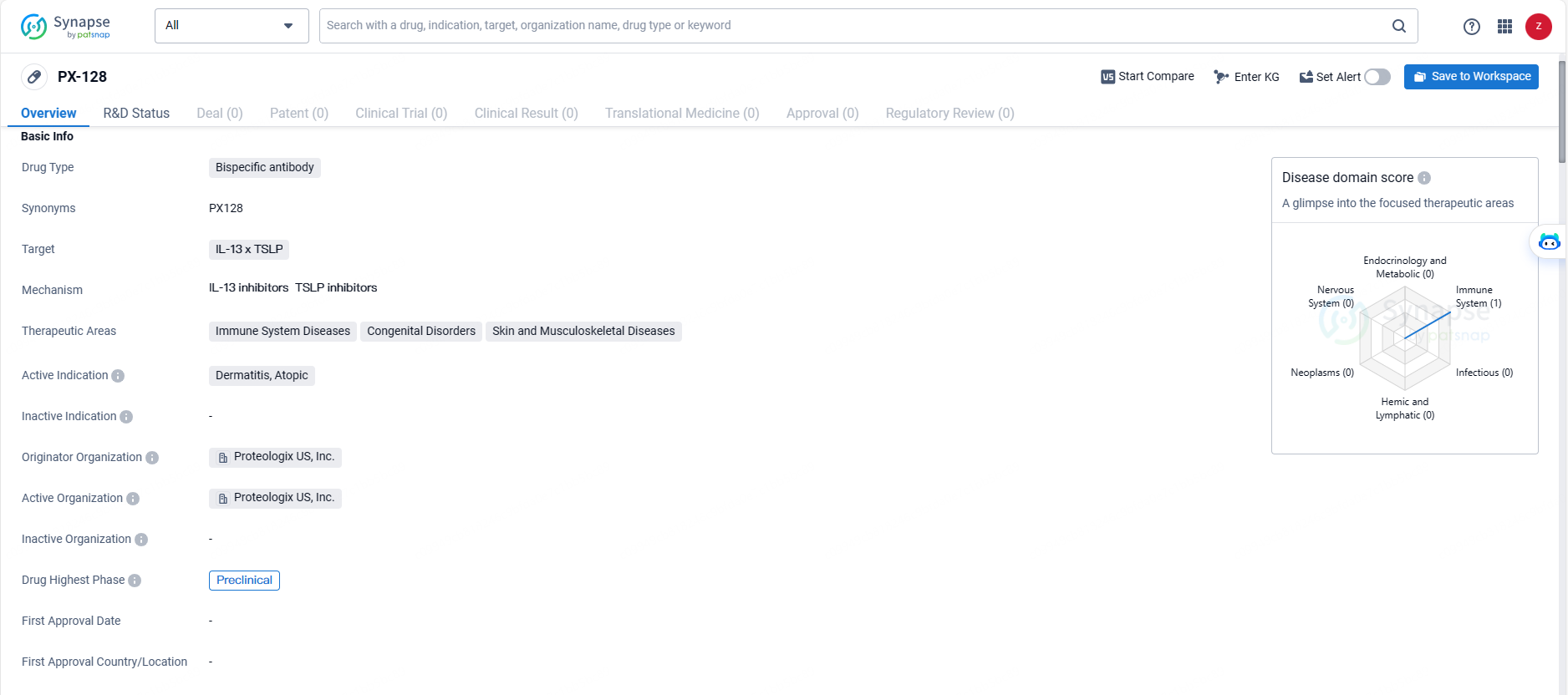
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Proteologix’s lineup includes PX128, a bispecific antibody that targets both IL-13 plus TSLP. PX128 is poised to advance into phase 1 development for the treatment of moderate to severe atopic dermatitis and asthma. Similarly, PX130, another bispecific antibody targeting IL-13 and IL-22, is in preclinical stages aimed at addressing moderate to severe atopic dermatitis.
Atopic dermatitis (AD) and asthma are heterogeneous conditions characterized by diverse pathogenic pathways across different patient groups. Therefore, targeting multiple pathways could enhance efficacy and potentially achieve remission. PX128 addresses IL-13-mediated Th2-driven skin inflammation, a pivotal pathway in both AD and asthma, and also targets TSLP, contributing to tissue inflammation in these diseases. Likewise, PX130 mitigates IL-13-mediated Th2 skin inflammation and additionally inhibits IL-22 to help restore the skin barrier and prevent inflammation from external factors like allergens. Both products are designed for less frequent dosing, offering greater convenience for patients. Collectively, these treatments highlight a strategic endeavor to build a portfolio of unique and synergistic bispecific antibodies.
Beyond PX128 and PX130, the acquisition will equip J&J with additional bispecific antibody programs applicable to a range of diseases, further enhancing the firm's capability to develop innovative bispecific therapies.
“Incorporating Proteologix bispecific antibodies into our portfolio marks a crucial initial step towards our commitment to individuals with AD,” stated Candice Long, Worldwide Vice President, Immunology, Johnson & Johnson. “We intend to broaden our impact, providing advanced and more targeted treatment options for those living with various immune-mediated conditions, thereby helping them achieve sustainable, symptom-free remission.”
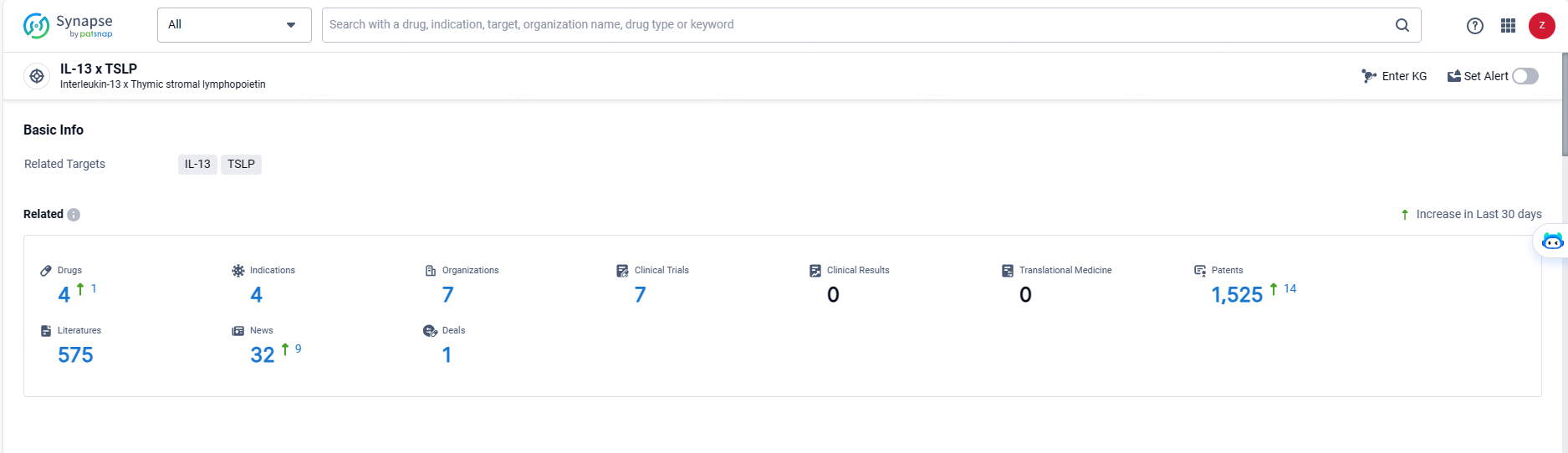
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 20, 2024, there are 4 investigational drugs for the IL-13 and TSLP targets, including 4 indications, 7 R&D institutions involved, with related clinical trials reaching 7, and as many as 1525 patents.
PX-128 is a bispecific antibody drug targeting IL-13 x TSLP, with a focus on treating atopic dermatitis. It is being developed by Proteologix US, Inc. and is currently in the preclinical phase of development. This drug has the potential to address unmet medical needs in the field of immune system diseases, congenital disorders, and skin and musculoskeletal diseases.