Johnson & Johnson Wins FDA and European Commission Nod for SIRTURO® (bedaquiline)
Johnson & Johnson revealed that the U.S. Food and Drug Administration has granted conventional approval for SIRTURO (bedaquiline) to be used as part of combination treatment for both adult and pediatric patients suffering from pulmonary tuberculosis caused by Mycobacterium tuberculosis resistant to a minimum of rifampicin and isoniazid. Following this FDA endorsement, the label limitations that were imposed when the drug received accelerated approval in the U.S. in December 2012 have been lifted.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The European Commission has granted full approval for SIRTURO, upgrading its Conditional Marketing Authorisation to a Standard Marketing Authorisation. This decision came after a favorable opinion from the European Committee for Medicinal Products for Human Use of the European Medicines Agency in April 2024.
The approvals relied on the results from the Phase 3 STREAM Stage 2 study, which stands as the first large-scale, randomized, multi-country open-label clinical trial to assess the efficacy and safety of an all-oral bedaquiline-containing regimen for treating MDR-TB. The study demonstrated that a bedaquiline-based regimen significantly improved treatment outcomes compared to those that included injectables. These findings were published in The Lancet in November 2022.
SIRTURO received accelerated approval from the FDA in December 2012 and conditional approval from the EMA in March 2014 based on positive Phase 2 study data. A supplemental New Drug Application was submitted to the FDA in August 2023, aimed at supporting its transition to full approval in the United States. Concurrently, a Type II variation was filed with the European Medicines Agency in November 2023 to support the shift to Standard Marketing Authorisation.
Johnson & Johnson has been dedicated to combating tuberculosis for years. When SIRTURO was introduced, it marked over four decades since the last TB treatment with a new mechanism of action was developed. It has become a fundamental part of the World Health Organization's recommended treatment guidelines for drug-resistant TB, with three-quarters of MDR-TB patients receiving an all-oral regimen containing bedaquiline. To date, over 845,000 courses of the medication have been distributed to 160 countries.
In 2023, Johnson & Johnson granted a license to the Stop TB Partnership’s Global Drug Facility, allowing it to tender, procure, and supply generic versions of SIRTURO to most low-and middle-income countries. Additionally, the Company announced its decision not to enforce bedaquiline patents in 134 low- and middle-income countries.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
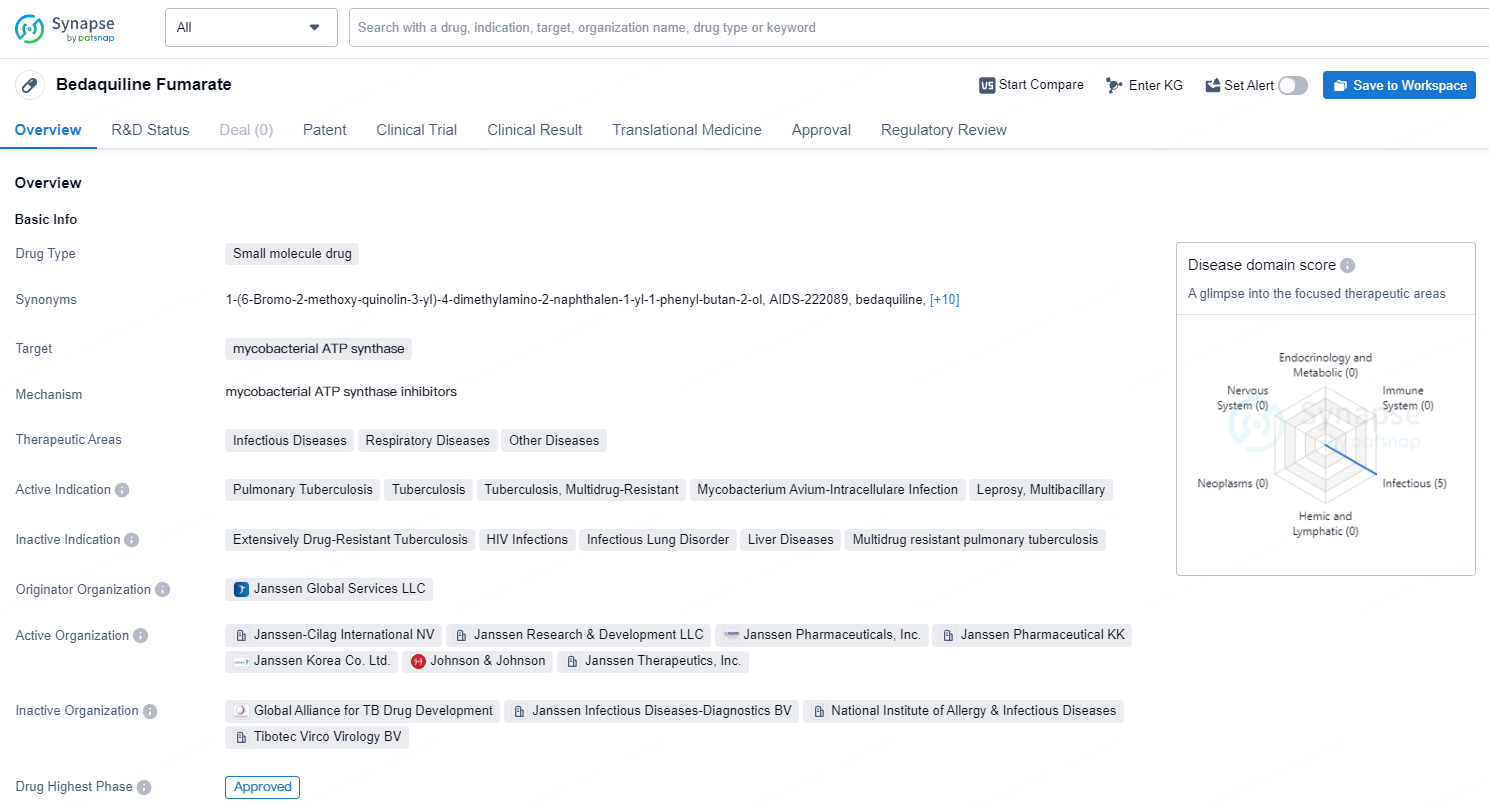
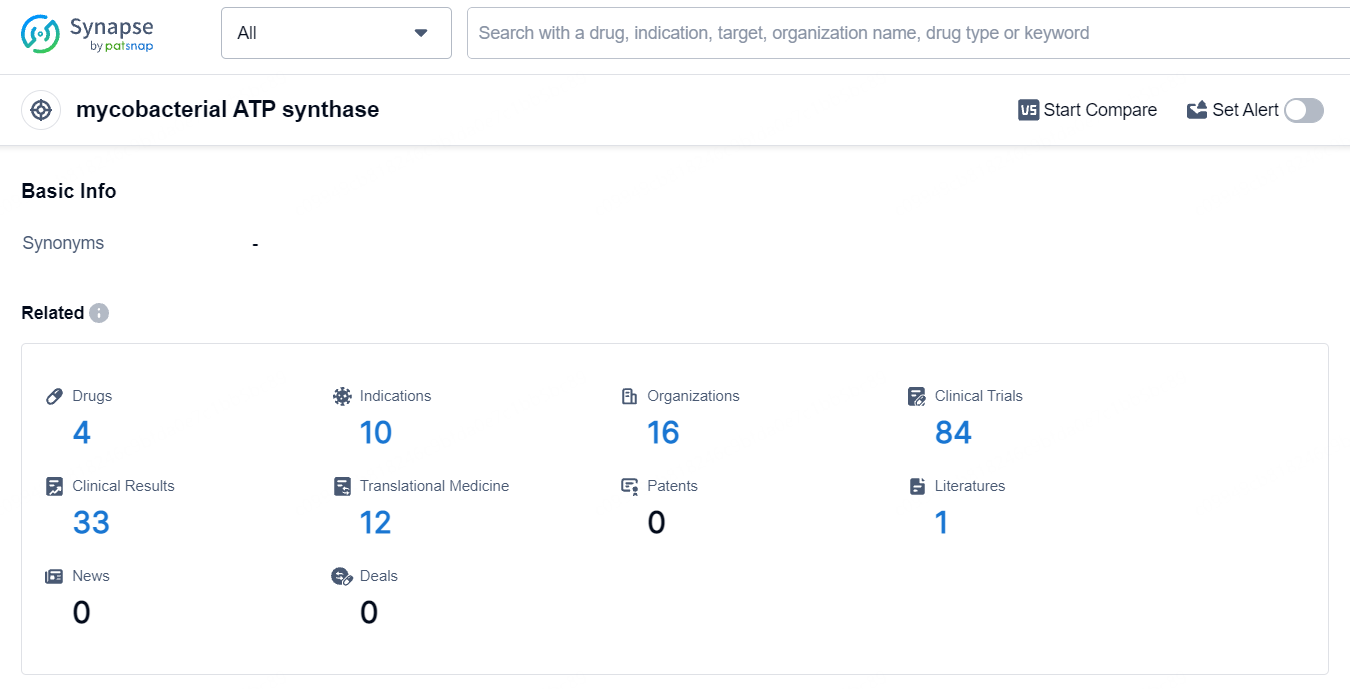
According to the data provided by the Synapse Database, As of July 8, 2024, there are 4 investigational drugs for the mycobacterial ATP synthase targets, including 10 indications, 16 R&D institutions involved, with related clinical trials reaching 84.
Bedaquiline Fumarate is a small molecule drug that targets mycobacterial ATP synthase and is used in the treatment of various infectious and respiratory diseases. As an expert in the pharmaceutical industry, it is important to closely monitor the market performance and any further developments related to Bedaquiline Fumarate, as it continues to play a crucial role in the management of these diseases.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!