Tyra Biosciences Reveals Preclinical Findings for TYRA-300 in Hypochondroplasia (HCH)
Tyra Biosciences, Inc., a biotechnology company in the clinical development phase that specializes in creating advanced precision therapies aimed at significant opportunities in the field of Fibroblast Growth Factor Receptor (FGFR) biology, has reported preclinical proof-of-concept findings for TYRA-300, an experimental orally administered FGFR3 selective inhibitor. These findings are related to hypochondroplasia.
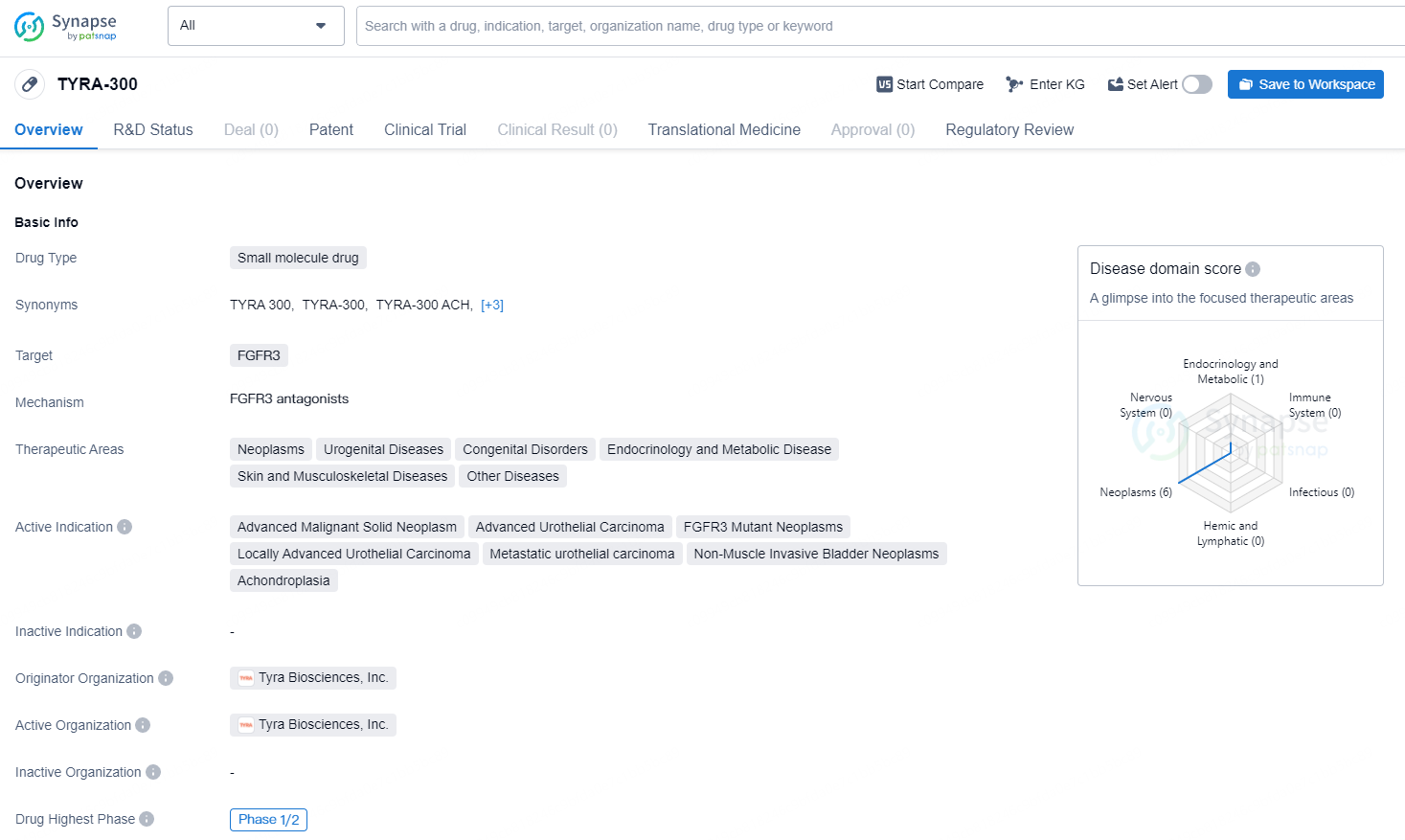
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Hypochondroplasia (HCH) is a form of skeletal dysplasia that is closely associated with achondroplasia, the most prevalent type of dwarfism. HCH most frequently arises from the N540K mutation in the FGFR3 gene. Presently, no approved treatments for HCH exist. The development of TYRA-300 aims to inhibit the mutation driving FGFR3-related skeletal dysplasias such as ACH and HCH.
“The recent preclinical data shared at Pharmachon 2024 are highly promising and bolster our confidence that TYRA-300 could emerge as a leading therapy addressing unmet medical needs in skeletal dysplasias,” stated Todd Harris, CEO of TYRA.
“Pursuing TYRA-300 for HCH is a logical progression from our efforts in ACH, and we are eager to file our IND in the latter half of this year to support our planned Phase 2 trial in pediatric achondroplasia,” Harris added.
“The enhancements in growth plate function noted in the hypochondroplasia mouse model offer compelling proof-of-concept evidence for the continued development of TYRA-300 in treating hypochondroplasia,” said Dr. Michael Bober, VP of Clinical Development and Medical Affairs at TYRA. “This further supports TYRA-300 as a potential targeted therapy for FGFR3-driven skeletal dysplasia.”
Susana Noval Iruretagoyena, Director of Fundación ALPE, remarked, “This is an exciting period for research into skeletal dysplasia, especially progressing into hypochondroplasia. Similar to achondroplasia, it will be crucial for companies to examine more than just height outcomes for these families.”
TYRA-300 is the Company’s primary precision medicine initiative, developed through its proprietary SNÅP platform. TYRA-300 is an investigational oral, FGFR3-selective inhibitor currently under development for treating cancer and skeletal dysplasias, including achondroplasia. In the field of oncology, TYRA-300 is undergoing evaluation in a multi-center, open-label Phase 1/2 clinical trial named SURF301 (Study in Untreated and Resistant FGFR3+ Advanced Solid Tumors), aimed at determining the recommended Phase 2 dose (RP2D) as well as assessing initial antitumor activity. In skeletal dysplasias, TYRA-300 has shown positive preclinical outcomes in both achondroplasia and hypochondroplasia, with plans to submit an IND in the second half of 2024 to commence a Phase 2 study in pediatric achondroplasia. The FDA granted Orphan Drug Designation (ODD) to TYRA-300 for the treatment of achondroplasia in July 2023, followed by Rare Pediatric Designation (RPD) in January 2024.
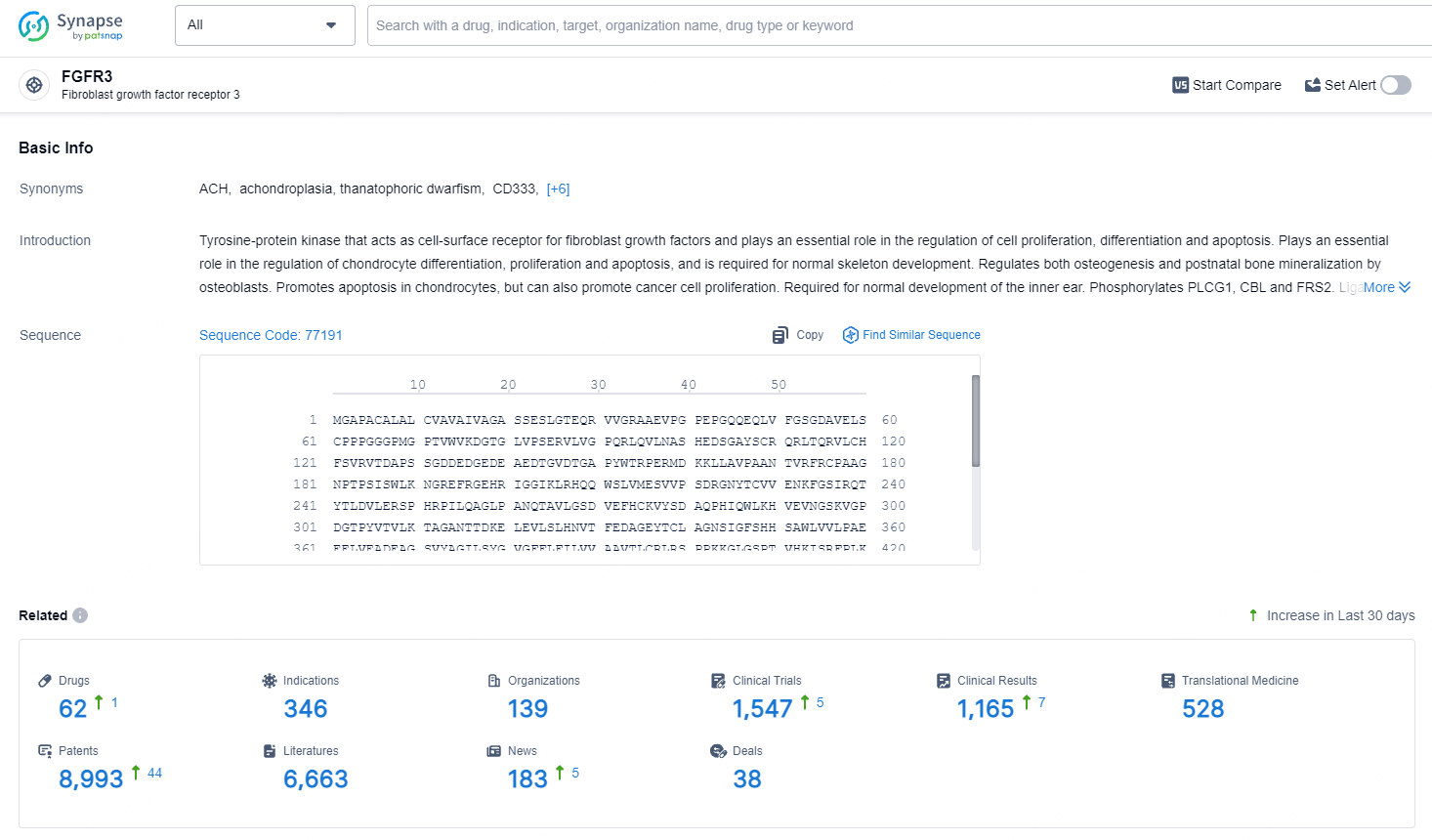
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 8, 2024, there are 62 investigational drugs for the FGFR3 target, including 346 indications, 139 R&D institutions involved, with related clinical trials reaching 1547, and as many as 8993 patents.
TYRA-300 is a small molecule drug developed by Tyra Biosciences, Inc. that targets FGFR3. TYRA-300 represents a promising small molecule drug with a diverse range of potential therapeutic applications, particularly in the treatment of neoplastic and urogenital diseases. Its designation for rare pediatric disease and orphan drug status further underscores its potential to address unmet medical needs in specific patient populations.