Kazia Therapeutics Presents EVT801 Findings at 15th Ovarian Cancer Conference
Kazia Therapeutics Limited (NASDAQ: KZIA), a company specializing in the development of cancer treatments, is delighted to report the presentation of data showcasing the significant clinical efficacy of EVT801 in treating high grade serous (HGS) Ovarian Cancer. This presentation occurred at the 15th Biennial Ovarian Cancer Research Symposium. The event was jointly organized by the American Association of Cancer Research (AACR) and the Rivkin Center for Ovarian Cancer Research, and took place on Saturday, September 21, 2024, in Seattle, Washington.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Dr. John Friend, CEO of Kazia Therapeutics, shared early results from a Phase 1 first-in-human clinical trial assessing the safety and tolerability of EVT801, a highly selective small molecule VEGFR3 inhibitor aimed at tumor angiogenesis. The Phase 1 trial achieved its main goals, determining the maximum tolerated dose at 500mg taken twice daily (BID). Additionally, this Phase 1 trial proposed a Phase 2 starting dose of 400mg BID. EVT801 was generally well-tolerated across all tested doses, with most side effects being mild to moderate and short-lived.
Highlights from the presentation included:
A total of 26 patients were treated over 6 different dosing levels, ranging from 50mg once daily (QD) to 500mg twice daily (BID)
Patients with a variety of eleven cancer types (e.g., colon, renal cell, pancreatic) participated, with advanced ovarian cancer, which had undergone significant prior treatments, being the most common (11 patients)
Biomarkers indicated a robust VEGFR3 presence in several cancer types, particularly ovarian cancer
Promising clinical effects were noted in High Grade Serous ovarian cancer patients, with 46% showing stable disease for at least three cycles, including two patients who completed 9 cycles
One patient experienced a partial response, evidenced by a 39% tumor reduction after 2 cycles of EVT801 therapy
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
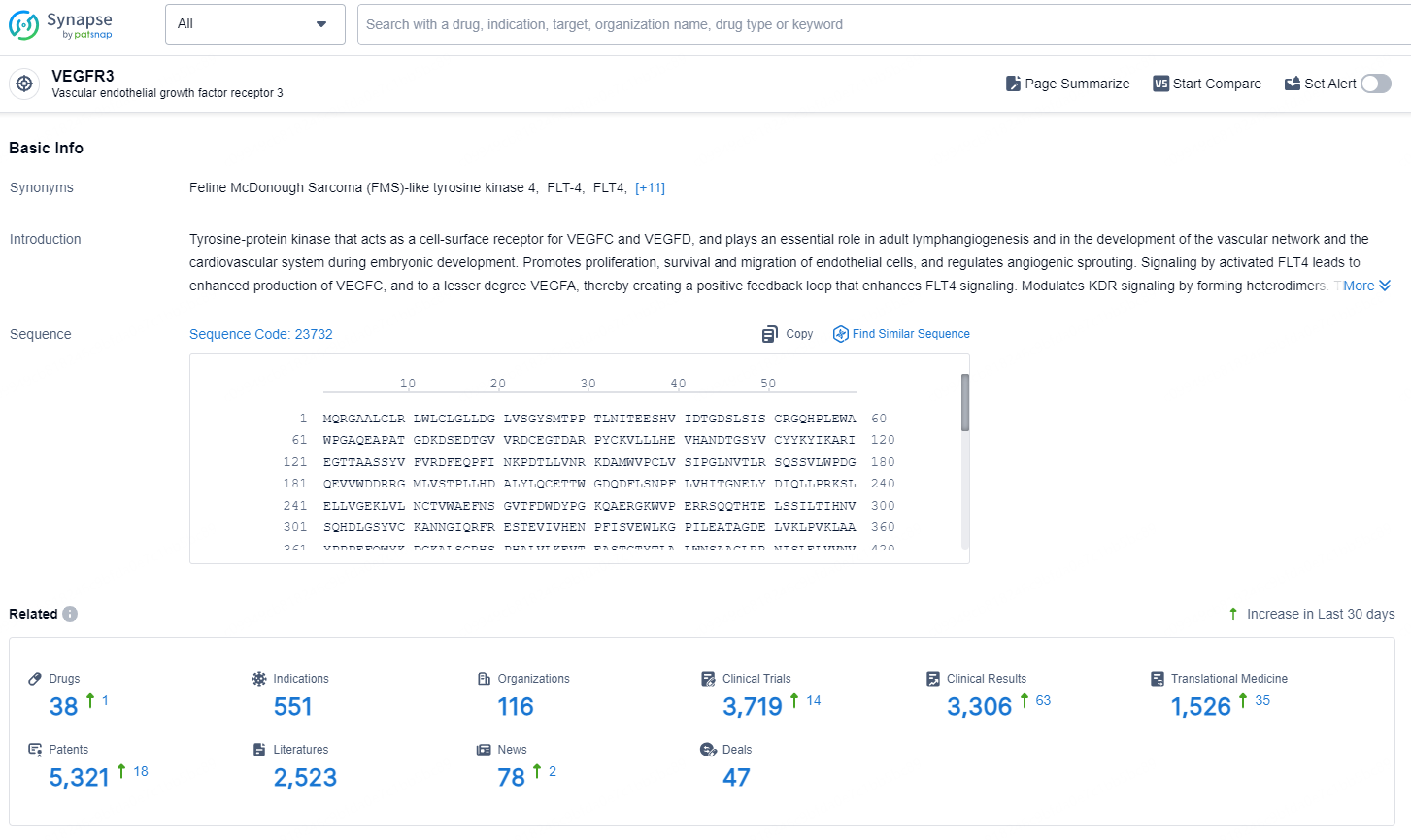
According to the data provided by the Synapse Database, As of September 24, 2024, there are 38 investigational drugs for the VEGFR3 target, including 551 indications, 116 R&D institutions involved, with related clinical trials reaching 3719, and as many as 5321 patents.
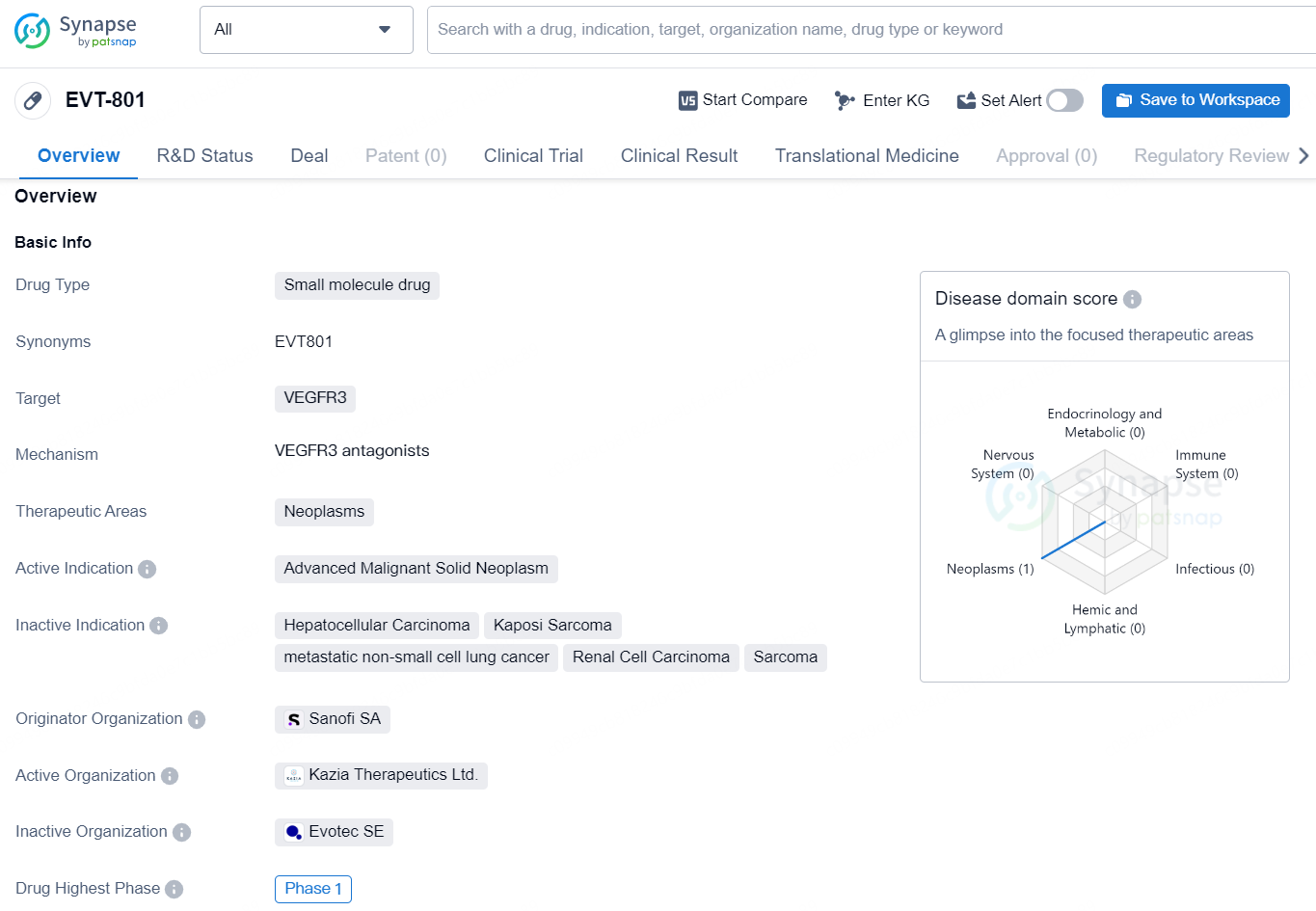
The small molecule drug EVT-801 is designed to target VEGFR3, a receptor commonly associated with neoplasms. The drug is intended for use in the treatment of advanced malignant solid neoplasms, indicating a focus on cancer therapy. Originating from Sanofi SA, EVT-801 has reached Phase 1 in its development, signifying that it has completed initial safety testing in humans and is now undergoing further clinical evaluation to assess its effectiveness and potential benefits for patients.