KEYTRUDA® Boosts DFS in High-Risk Urothelial Carcinoma Post-Surgery
The pharmaceutical giant Merck, also recognized as MSD in regions other than the United States and Canada, recently unveiled the outcomes from its Phase 3 AMBASSADOR /KEYNOTE-123 study. This critical trial assessed the efficacy of KEYTRUDA, the company's proprietary anti-PD-1 treatment, when administered as an adjunct therapy for patients with high-risk localized muscle-invasive urothelial carcinoma, as well as those with resectable locally advanced urothelial carcinoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
 Chief of the Genitourinary Malignancies Branch at the National Cancer Institute, which is integrated into the National Institutes of Health, Dr. Andrea Apolo, reported that despite aggressive surgical intervention to excise all malignant growths, around 50% of those with muscle-invasive bladder cancer still face a significant likelihood of the disease returning.
Chief of the Genitourinary Malignancies Branch at the National Cancer Institute, which is integrated into the National Institutes of Health, Dr. Andrea Apolo, reported that despite aggressive surgical intervention to excise all malignant growths, around 50% of those with muscle-invasive bladder cancer still face a significant likelihood of the disease returning.
Dr. Apolo elaborated, "Our study revealed that adjuvant therapy with pembrolizumab managed to lower the chances of recurrence or mortality by 31% when compared to mere surveillance. This underscores the efficacy of pembrolizumab post-operatively in patients at heightened risk—those with lingering muscle-invasive or advanced local stages of urothelial carcinoma who exhibit a high tumor grade, possess lymph nodes bearing the disease, or show evidence of cancer at the surgical margins—assisting in impeding the resurgence of cancer."
Dr. Marjorie Green, who holds the position of senior vice president and leads the oncology clinical development worldwide at Merck Research Laboratories, commented, "The Phase 3 findings are groundbreaking, revealing KEYTRUDA's first significant positive outcome in DFS when used as an adjuvant treatment for urothelial carcinoma. The critical conclusions of this study position KEYTRUDA as a promising adjuvant treatment pathway for such individuals and highlight its growing applicability in the initial treatment phases of resectable muscle-invasive bladder cancer."
Merck is actively expanding its investigational endeavors surrounding KEYTRUDA, examining its potential both alone and in synergistic use with other cancer-fighting agents for various stages of bladder cancer, ranging from non-muscle-invasive through muscle-invasive to metastatic forms. The Phase 3 KEYNOTE-866 trial is a part of this expansive program, complemented by the KEYNOTE-B15 and KEYNOTE-905 Phase 3 studies, which are conducted in strategic alliance with Pfizer and Astellas.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
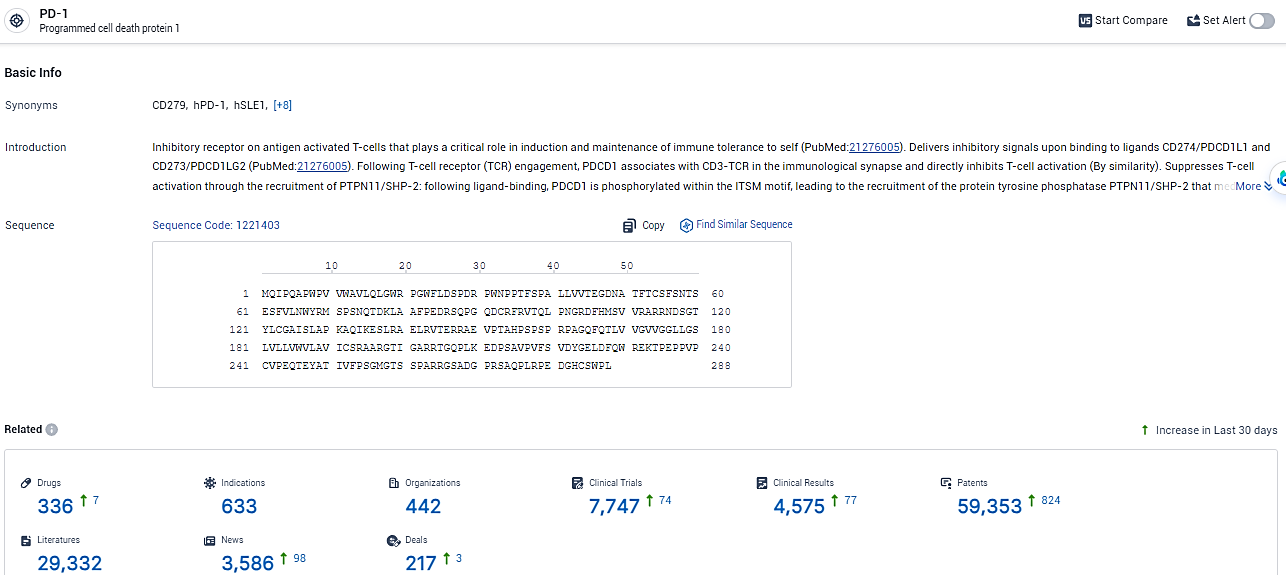
According to the data provided by the Synapse Database, As of January 31, 2024, there are 336 investigational drugs for the PD-1 target, including 633 indications, 442 R&D institutions involved, with related clinical trials reaching 7747, and as many as 59353 patents.
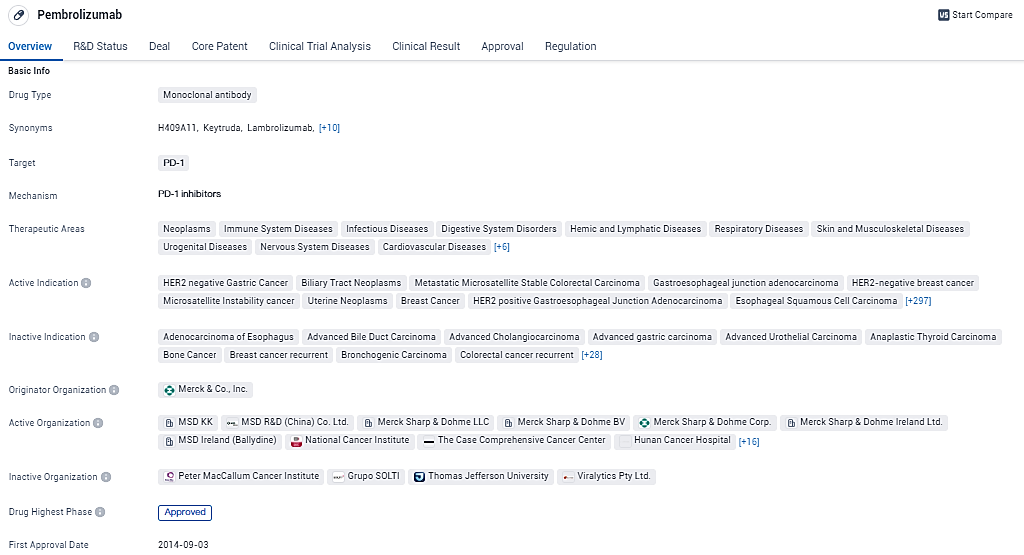
Pembrolizumab is an anti-programmed death receptor-1 therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. Pembrolizumab is a monoclonal antibody drug that targets PD-1 and has been approved for the treatment of various cancers and other diseases. Its broad therapeutic areas, multiple approvals, and special designations demonstrate its potential to revolutionize the treatment landscape in the pharmaceuticals.