KRASG12D-LODER: brief review of its R&D progress and the clinical result in 2023 ESMO
Locally advanced pancreatic cancer (LAPC) accounts for 30% of pancreatic cancer (PC). LAPC incurs high mortality. On October 20, 2023, a novel extended-release siRNA targeting KRASG12D/V (G12D/V) mutations (Loder) in combination with standard chemotherapy was reported at the ESMO Congress.
KRASG12D-LODER's R&D Progress
The drug KRASG12D-LODER is a small interfering RNA (siRNA) that targets the KRAS G12D gene. It is being developed for the treatment of pancreatic cancer, a type of neoplasm that affects the digestive system. Additionally, it has potential therapeutic applications in the fields of endocrinology and metabolic diseases.
According to the Patsnap Synapse, KRASG12D-LODER is currently in Phase 2, which is the second highest phase in the drug development process. And the clinical trial areas for KRASG12D-LODER are primarily in the United States and Israel. The key indication is Pancreatic Ductal Adenocarcinoma and Locally Advanced Pancreatic Adenocarcinoma.
Detailed Clinical Result of KRASG12D-LODER
The two-cohort, phase II multicenter, open-label trial (NCT01676259) was conducted in pancreatic cancer patients.
In this study, cohort 1: Patients (pts) with LAPC randomized to: Loder + gemcitabine/nab-paclitaxel (GnP); or GnP. Cohort 2: pts with Borderline Resectable (BR) PC or LAPC single arm, Loder + (modified)FOLFIRINOX ((m)FFX) or GnP. The Loder was inserted into primary tumor via endoscopic ultrasound (EUS 19G needle) q3 mo. for 2-3 doses. mITT, all patients ≥ 1 treatment. Key study endpoints: overall survival (OS), safety.
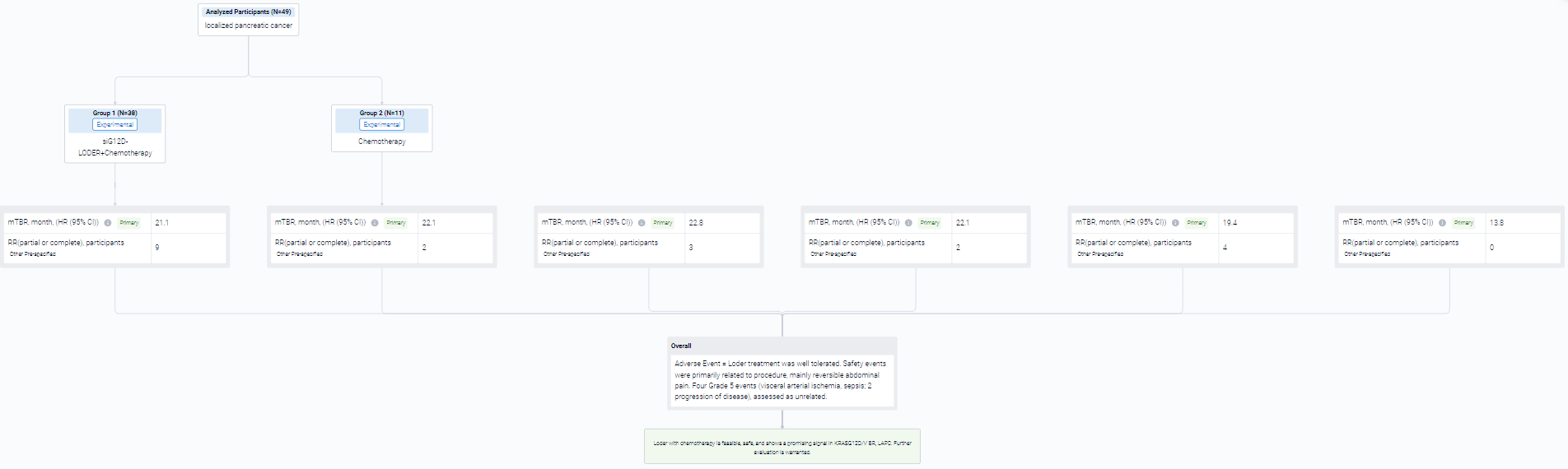
The result showed that N= 49 pts in mITT (6 BRPC, 43 LAPC, Loder n=38, control n=11). KRAS known for n=35: G12V, n=12; G12D, n=11; G12R, n=7; Q61R, n=2; wild-type, n=1. For mITT: no difference in OS for Loder + chemo vs. chemo (OS=21.1 vs. 22.2 mo.). However, in pts with G12D/V a non-statistically significant numerical advantage was seen in Loder + (m)FFX or GnP arm (n=18, Cohorts 1,2) median OS = 19.4 mo. vs. 13.8 mo.
It can be concluded that loder with chemotherapy is feasible, safe, and shows a promising signal in KRASG12D/V BR, LAPC. Further evaluation is warranted.
How to Easily View the Clinical Results Using Synapse Database?
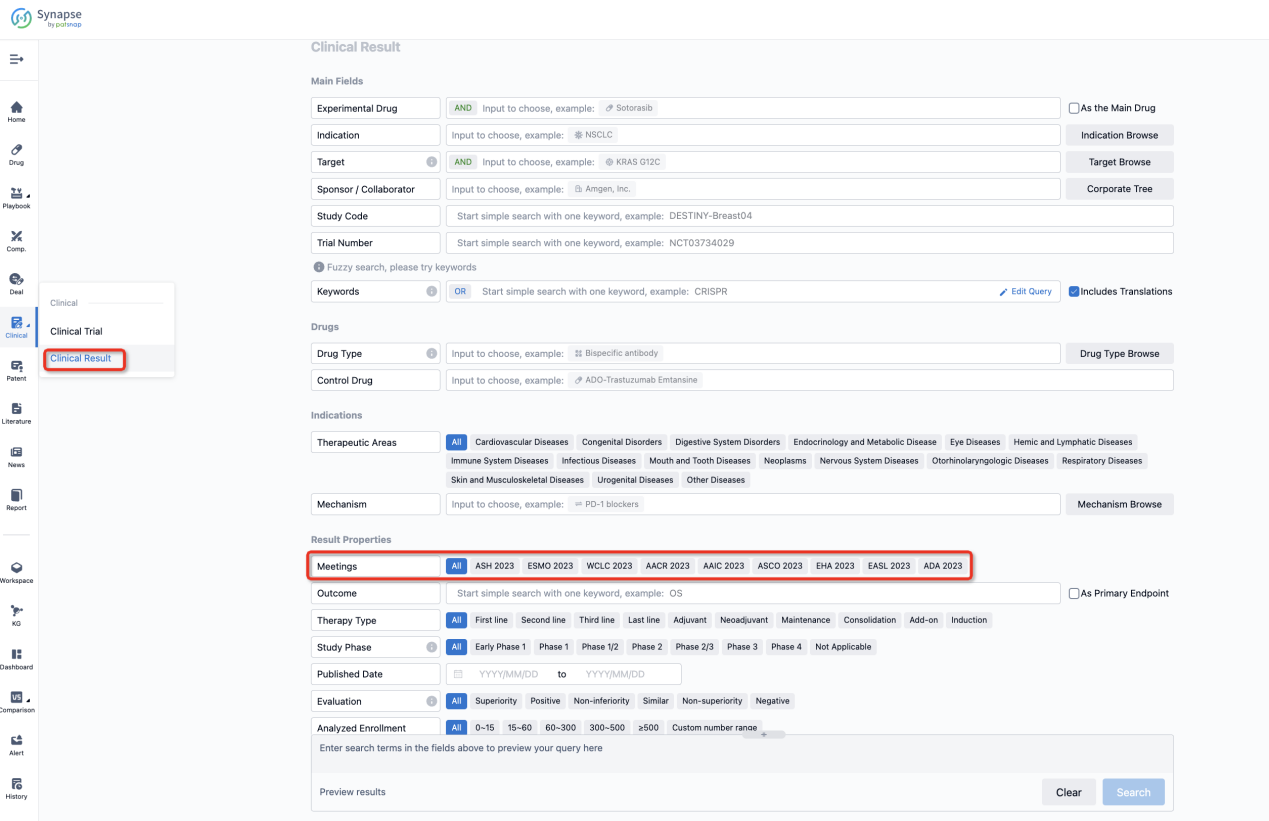
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
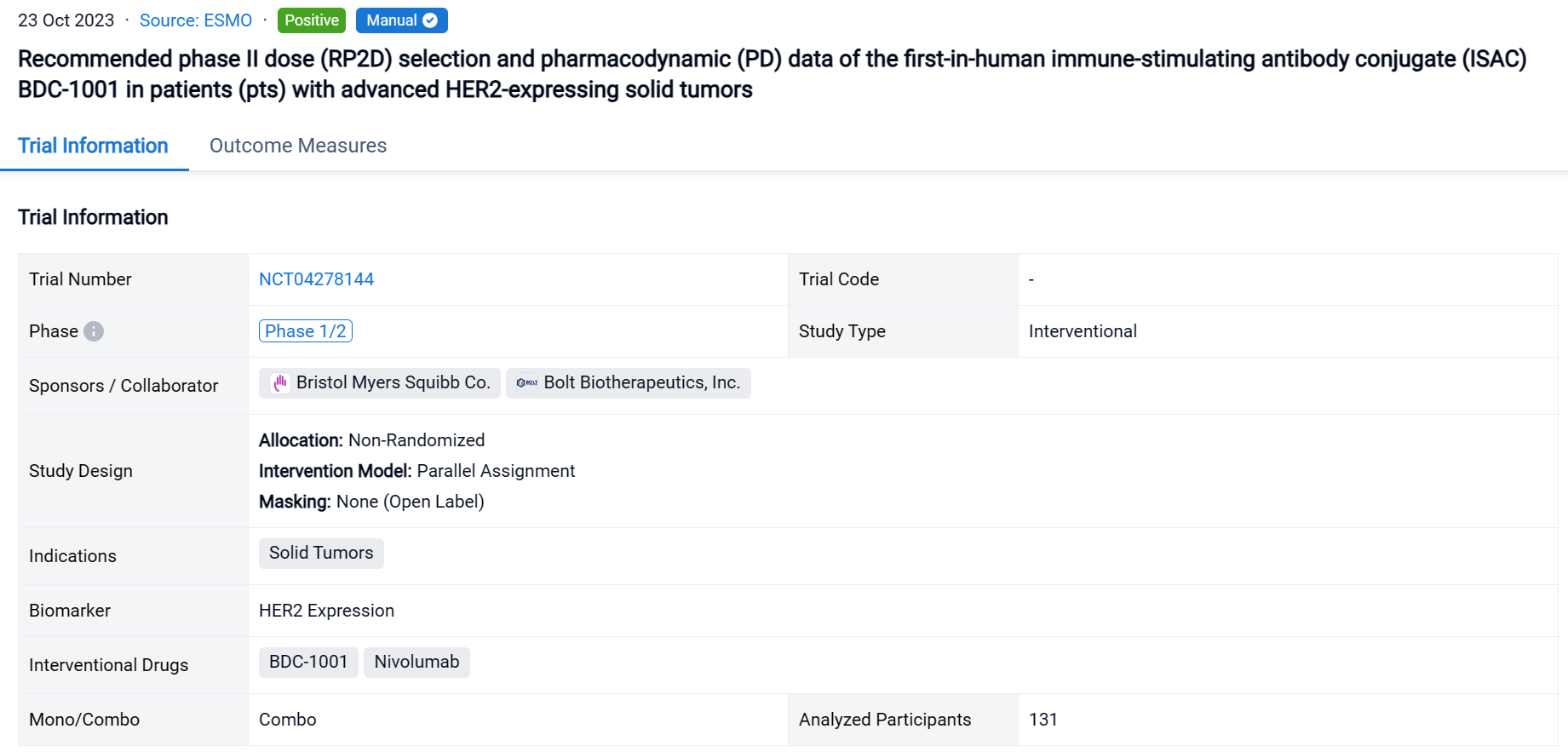
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
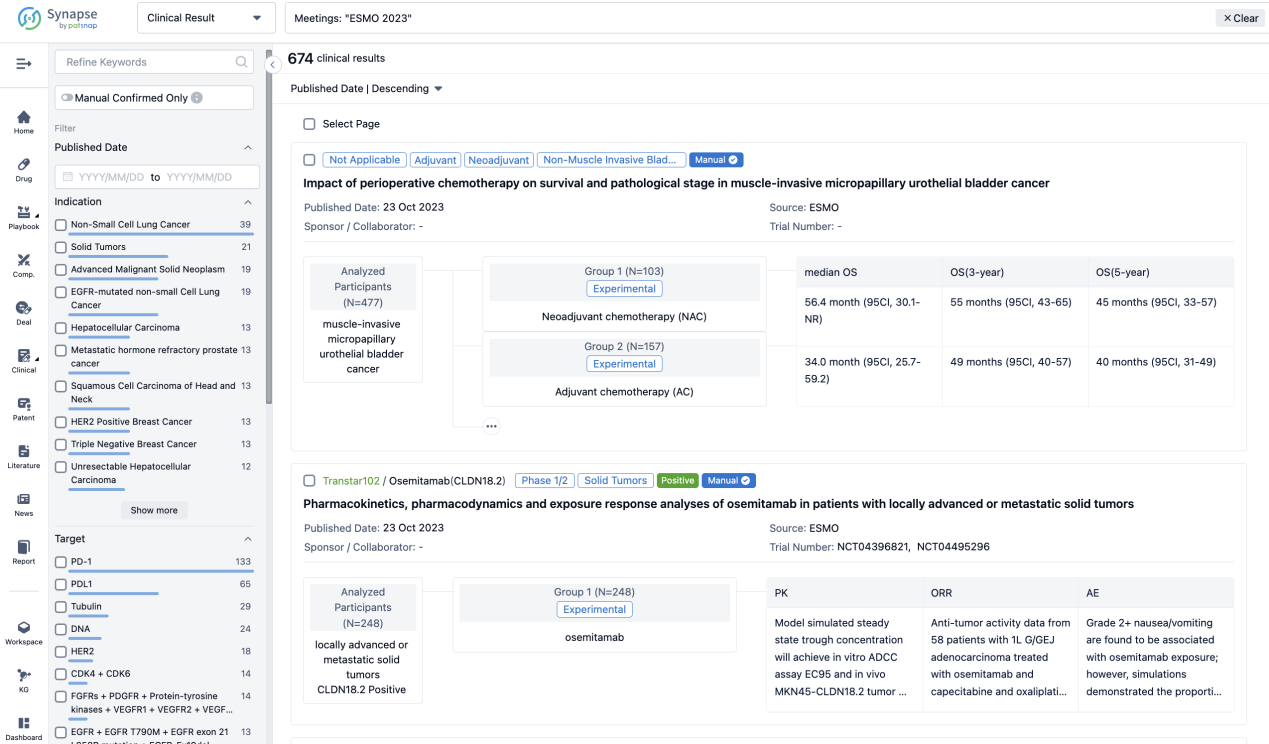
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.


Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!