KYV-101 Receives FDA IND Approval for Phase 2 KYSA-8 Study in Treatment-Resistant Stiff-Person Syndrome
Kyverna Therapeutics, Inc., a clinical-stage biopharmaceutical company dedicated to creating cell therapies for patients with autoimmune conditions, announced today that the U.S. Food and Drug Administration has approved its Investigational New Drug application. This approval pertains to KYV-101, an autologous, fully human anti-CD19 chimeric antigen receptor T-cell product candidate, intended for treating stiff-person syndrome in Kyverna’s clinical trial designated KYSA-8.
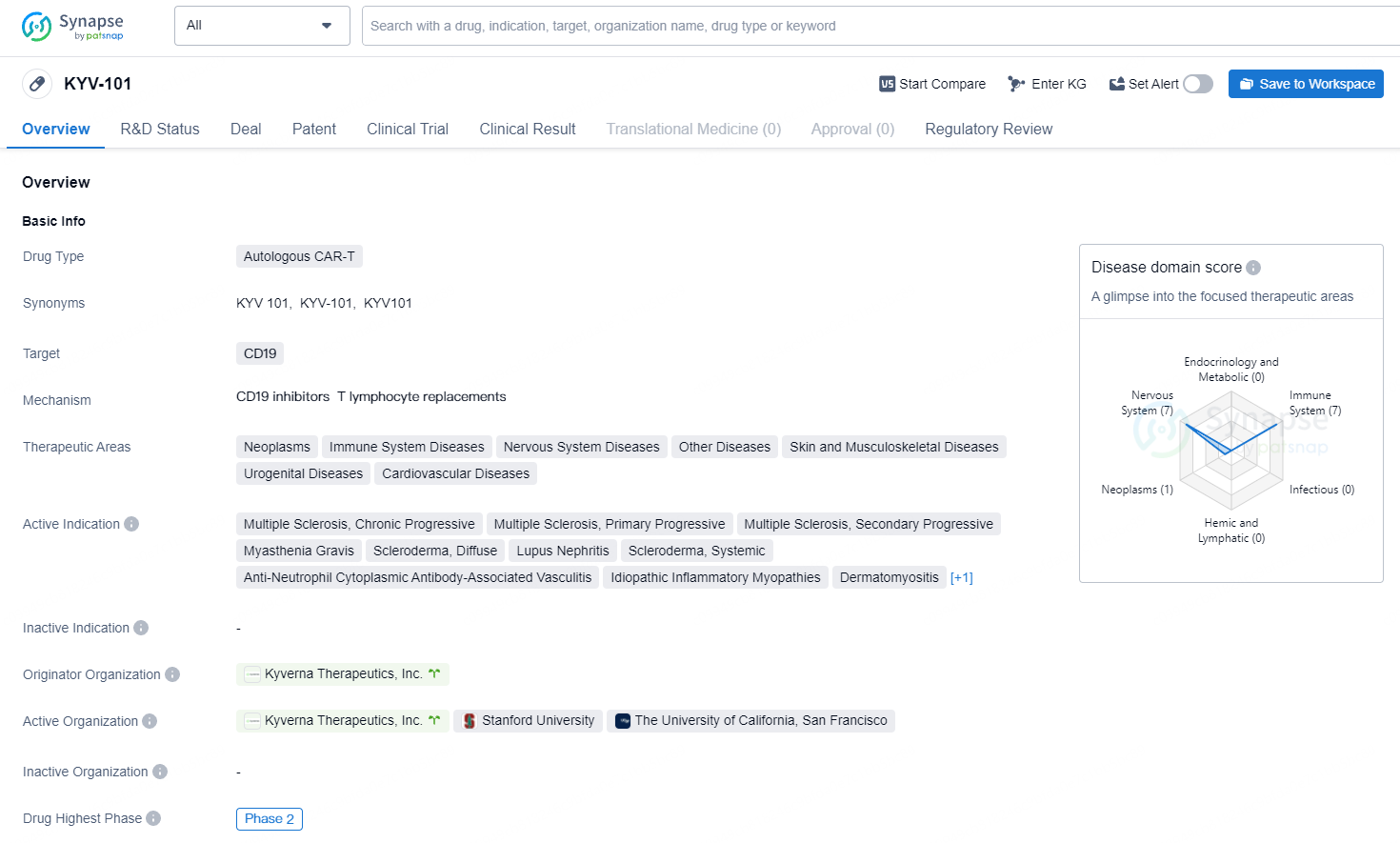
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"CAR T-cell therapy has already shown initial but encouraging results in patients with SPS treated outside the United States," stated Marinos Dalakas, M.D., FAAN, Professor of Neurology, and Director of the Neuromuscular Division at Thomas Jefferson University School of Medicine in Philadelphia, PA, a leading authority on SPS. "I consider the KYSA-8 trial to be of significant importance as an innovative promising therapy for patients with stiff person syndrome who do not respond to existing treatments, suggesting the potential for prolonged benefits."
"The IND clearance strengthens our commitment to potentially revolutionizing the treatment for patients affected by SPS and confirms a target dose of 100 million cells for KYV-101," commented Sham Dholakia, M.D., business unit head for rare diseases at Kyverna. "We deeply appreciate the FDA's collaborative approach and prompt evaluation of our clinical trial design."
SPS is a rare, progressive neurological autoimmune disorder characterized by severe muscle stiffness in the torso, arms, and legs, which impairs the ability to walk or move. Patients typically exhibit muscle spasms and stiffness, making movements such as turning and bending difficult. In severe cases, the patient's walking can appear statue-like. Muscle spasms and stiffness can be triggered by unexpected stimuli, such as loud sounds, sudden touches, or anxiety-inducing situations, which may be mistakenly diagnosed as primary anxiety disorders.
KYV-101 is a fully human, autologous anti-CD19 CAR T-cell therapy candidate designed for B cell-mediated autoimmune diseases. Developed by the National Institutes of Health, the CAR in KYV-101 was engineered to enhance tolerability and was tested in a Phase 1 trial involving 20 cancer patients. The results were published by the NIH in Nature Medicine.
KYV-101 is currently under evaluation in sponsored, open-label Phase 1/2 and Phase 2 trials in the United States and Germany, addressing two major areas of autoimmune disease: rheumatology and neurology.
With 50 patients having received the CAR in KYV-101 for both oncological and autoimmune conditions across over 15 sites in Europe and the U.S., we believe the distinct properties of KYV-101 are vital for the potential success of CAR T-cell therapies in treating autoimmune diseases.
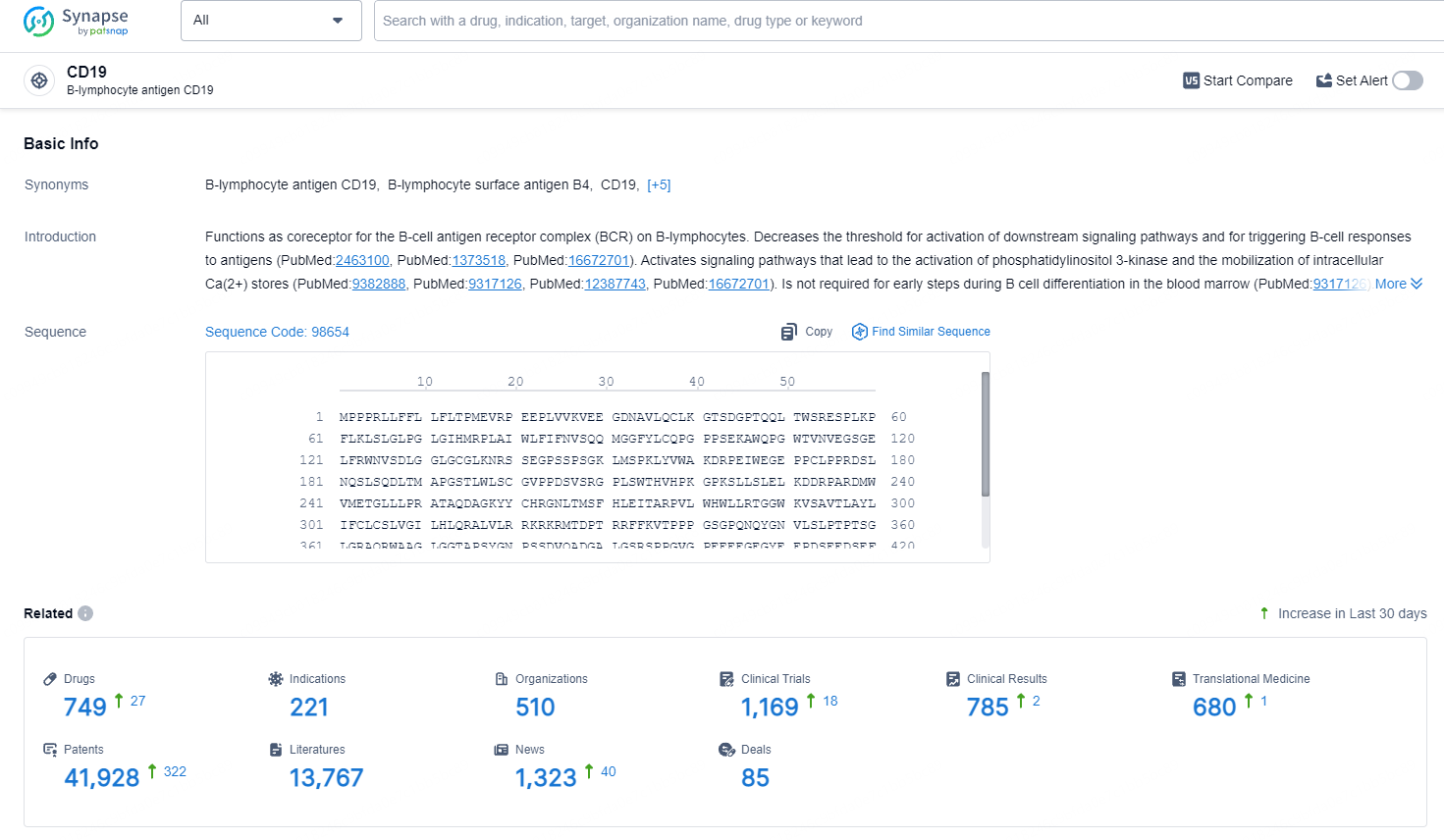
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 26, 2024, there are 749 investigational drugs for the CD19 target, including 221 indications, 510 R&D institutions involved, with related clinical trials reaching 1167, and as many as 41931 patents.
KYV-101 targets CD19 and is being developed for the treatment of various therapeutic areas including neoplasms, immune system diseases, nervous system diseases, skin and musculoskeletal diseases, urogenital diseases, and cardiovascular diseases. KYV-101 is currently in the highest phase of development, which is Phase 2, on a global scale. The drug has been granted Fast Track and Orphan Drug designations, indicating that it is intended for the treatment of serious or rare conditions and may receive expedited review and approval from regulatory authorities.