LaNova Medicines Initiates Phase 1 Trial for Bispecific Antibody LM-299 and Secures $42M in Series C1 Funding
LaNova Medicines Limited, a privately-owned biotech focused on innovation and currently in the clinical phase, is specializing in antibody-drug conjugates (ADCs) and immuno-oncology. The Company has revealed the start of its Phase 1 clinical trial for LM-299, a bispecific antibody targeting PD-1/VEGF, in China aimed at treating advanced solid tumors. Additionally, it has successfully concluded its Series C1 financing round, raising $42 million.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Established in September 2019, LaNova's research and development framework operates on three unique platforms designed to address difficult therapeutic targets and support diverse modality innovation. To date, this has led to the internal creation of over ten groundbreaking programs, which encompass monoclonal antibodies, antibody-drug conjugates (ADCs), and bispecific antibodies.
After receiving encouraging preclinical outcomes showing that LM-299 effectively inhibits tumor growth in human peripheral blood mononuclear cells (hPBMCs)-humanized mice and demonstrates a favorable safety profile in non-human primate (NHP) GLP toxicity studies, LaNova has commenced its first clinical trial in humans in China for advanced solid tumors. Additionally, the company plans to launch another Phase 1 clinical trial in the United States, with an Investigational New Drug (IND) application anticipated to be filed in the latter half of 2024.
The recently concluded Series C1 funding round was spearheaded by Sino Biopharmaceuticals, with contributions from new investors such as Pudong Innovation Investment and Zhangjiang Haoheng, along with existing backers including Qiming Venture Partners and Shanghai Healthcare Capital. Zhong Lun Law Firm served as the legal counsel for this financing round.
LaNova has now started its Series C2 funding initiative, with the proceeds primarily aimed at furthering the clinical advancement of its pipeline, particularly the leading candidates:
- LM-302 (anti-CLDN 18.2 ADC): Currently undergoing a Phase III registration trial in China for gastric cancers, positioning it as one of the three most advanced candidates globally for this indication, with a Phase II trial in the US projected to commence in the second half of 2025.
- LM-108 (anti-CCR8 monoclonal antibody): Active Phase II clinical trials in China for various solid tumors make it one of the leading global projects targeting CCR8, with a US Phase II trial expected to begin in the second half of 2024.
- LM-299 (anti-PD-1/VEGF bispecific antibody): A Phase I clinical trial is currently in progress in China, enrolling patients with advanced solid tumors.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 23, 2024, there are 15 investigational drug for the PD-1 and VEGF targets, including 36 indications, 21 R&D institutions involved, with related clinical trials reaching 387, and as many as 9242 patents.
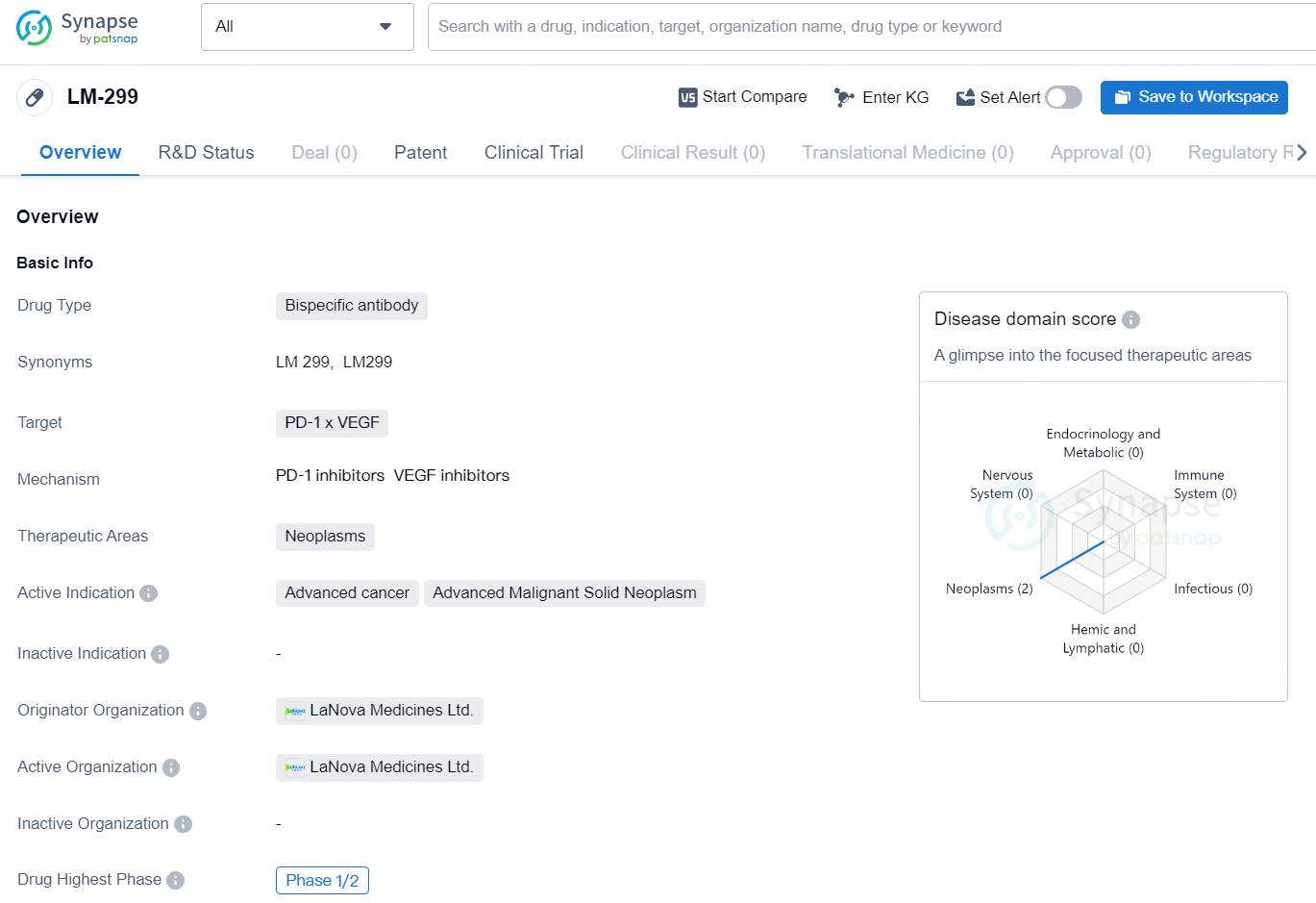
LM-299 is a bispecific antibody drug developed by LaNova Medicines Ltd. The drug targets the PD-1 and VEGF proteins and is intended for use in the treatment of neoplasms, specifically advanced cancer and advanced malignant solid neoplasm. The highest phase of development for LM-299 globally is Phase 1/2, indicating that it has progressed beyond the initial preclinical and Phase 1 stages of testing. In China, the highest phase achieved is also Phase 1/2, suggesting that the drug is currently undergoing clinical trials in this region.