Latest Competitive Analysis of AbbVie Drug Pipeline
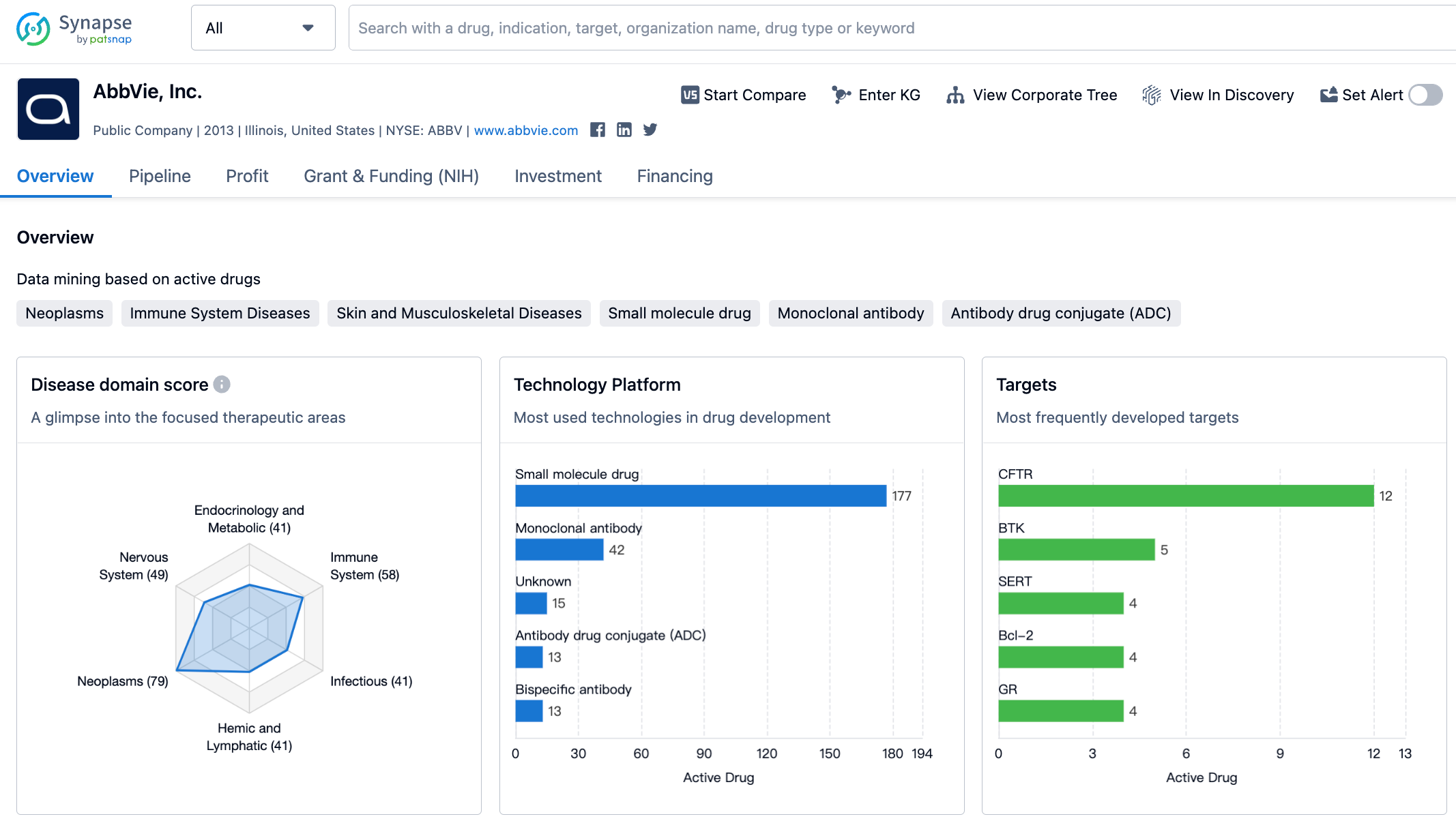
Since acquiring Allergan, AbbVie has experienced a period of stable performance, but this year has seen a significant decline in the performance of its two heavyweight drugs, Adalimumab and Ibrutinib. Fortunately, the newer generation drugs such as Risankizuma, Upadacitinib, Venetoclax, and Cariprazine have made a certain contribution to growth, so the overall performance has not been too bad, which is why the market value has generally remained around 250 billion.
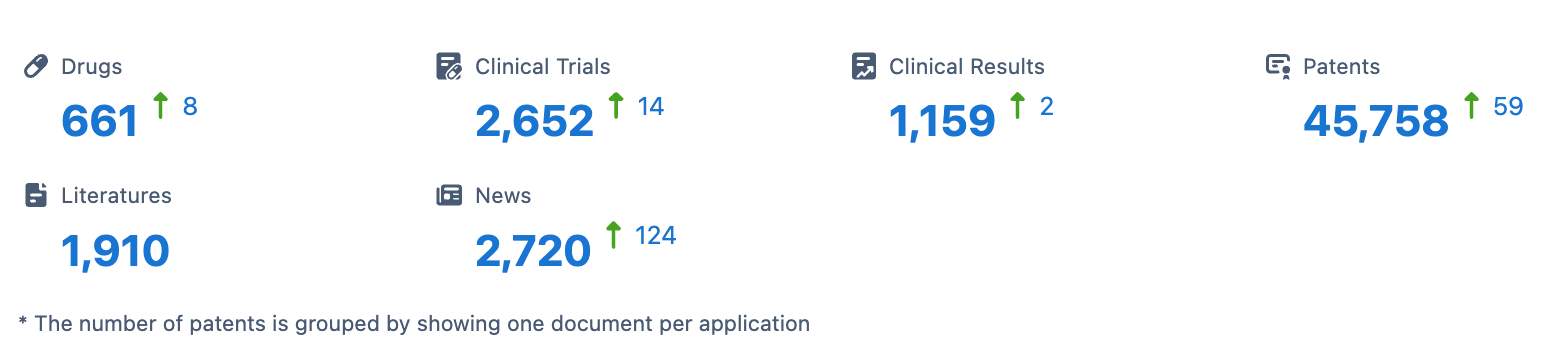
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of AbbVie.
In terms of R&D, there have not been many surprises. The best news includes CD20×CD3 bispecific antibody Epcoritamab getting approval, as well as daily dose formulation of JAK1 inhibitor Upadacitinib being approved, phase 3 results for IL23p19 monoclonal antibody Risankizumab's UC and Ps indications, and phase 3 results for migraine drug Atogepant.
In terms of bad news, aside from Ibrutinib withdrawing MZL&MCL indications, TNFα ADC drug ABBV-154 and RORγt inverse agonist Cedirogant have both been terminated, while the Parkinson's combination drug ABBV-951 has received a Complete Response Letter (CRL) from the FDA.